Abstract
In indoor environments, cooking is a major contributor to indoor air pollution releasing potentially harmful toxic compounds such as polycyclic aromatic hydrocarbons. In our study, Chlorophytum comosum ‘Variegata’ plants were applied to monitor PAH emission rates and patterns in previously selected rural Hungarian kitchens. Concentration and profile of accumulated PAHs could be well explained by cooking methods and materials used in each kitchen. Accumulation of 6-ring PAHs was characteristic in the only kitchen which frequently used deep frying. It also should be emphasized that applicability of C. comosum as indoor biomonitor was assessed. The plant has proven a good monitor organism as it accumulated both LMW and HMW PAHs.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
In Europe, people spend approximately 90% of their time indoors (González-Martín et al. 2021), which has raised the necessity to investigate indoor air pollution levels and their potential health impacts. Most studies agree that cooking is a major contributor to indoor air pollution (Zhai and Albritton 2020). During cooking, particulate matter (PM) can arise from the food source, fuel used and from the coating layers of heated cookware (Cho et al. 2019). Considering the number of emitted particles, the dominant fractions are ultrafine particles (UFPs) and accumulation mode particles (AMPs) (Li et al. 2017a). In the study of Wallace and Ott (2011), cooking was one of the greatest indoor sources of ultrafine particles (UFP). The smaller the more dangerous: smaller particles bind relatively more potentially toxic compounds due to the relatively bigger surface. Also, submicron particles can penetrate the respiratory system deep causing serious health risks (Zhang et al. 2017).
Deep-frying is one of the most prevailing cooking methods (Yao et al. 2015, Liu et al. 2019, Touffet et al. 2020). Most studies agree that frying produces higher particle emissions compared to steaming and boiling (reviewed by Torkmahalleh et al. 2017). It is usually conducted at high temperatures (150 to 190 °C) which favors the emission of polycyclic aromatic hydrocarbons (PAHs). In many households, the same batch of oil is used on several occasions which further increase the risk of PAH emissions (Ng et al. 2014, Hao et al. 2016, Juániz et al. 2016). Iwegbue et al. (2020) measured PAH content in different vegetable oils before use and after three successive cycles of frying, reporting significant increases. Raw material can also influence emission rates, for example preparing fatty meats generate higher emissions than vegetables (reviewed by Li 2021).
Several studies have reported the dominance of naphthalene (Nap) in different kitchens (e.g., Zhu and Wang 2003, Wang et al. 2015). Nap mainly occurs in the gaseous phase; its relative share might reach as much as 89% of the total gaseous PAHs (Chen et al. 2007). It was for example detected in the breathing area of kitchen workers (Sjaastad and Svendsen, 2009). Huang et al. (2021) compared volatile organic compounds emission in frying, steaming, and grilling commercial kitchens and found that the three cooking styles had similar production of Nap. Its metabolite 2-hydroxynaphthalene was also detected in the urine sample of kitchen workers, indicating oxidative stress. It is a two-ring, low molecular weight PAH which is considered an ubiquitous environmental pollutant (Preuss et al. 2003). Based on the degree of evidence for the carcinogenicity, the International Agency for Research on Cancer (IARC) identified naphthalene as “possibly carcinogenic to humans” (group 2B) (IARC 2010).
While active sampling has been widely applied to characterise the risk of PM and especially PAH emissions during cooking, biomonitoring studies have been mostly restricted to human samples (Li et al. 2011, Oliveira et al. 2017, Murawski et al. 2020). Neupane et al. (2019), e.g., used hair samples of cooks to estimate health risk of chronic exposure in restaurants. Considering non-human samples, spider webs were used in the studies of Rutkowski et al. (2019) and Rybak et al. (2019) to monitor indoor PAH levels. Spider webs can be collected in situ (van Laaten et al. 2020), while other traditional biomonitors such as lichens need to be transplanted. Transplanted Pseudovernia furfuracea plants were used in the study of Protano et al. (2017) in indoor school environments. Lichen samples accumulated both heavy metals and PAHs. Transplanted lichens, however, might face a certain level of physiological stress caused by specific conditions such as temperature and humidity (Canha et al. 2012). Paoli et al. (2019) placed Evernia prunastri in school environments and found that in two months exposure physiological impairment of the samples could be avoided, and this time was sufficient for the test plants to bioaccumulate heavy metals.
Biomonitors are considered a cheap and viable solution for monitoring and show adequate sensitivity even in case of relatively low contamination level (Baldantoni and Alfani 2016). Results will provide a time-averaged data series (Zhao et al. 2018). It might be especially important in kitchen environments where emission intensity can show high temporal variations: for example, fine particulate matter (PM2.5) concentrations increased by a factor of as much as 85 during cooking compared to non-cooking time in the study of See and Balasubramanian (2006).
In our study, Chlorophytum comosum ‘Variegata’ (Thunb.) Jacques (Family Asparagaceae, spider plant, syn. spider ivy, ribbon plant, or hen and chickens) plants were applied to monitor PAH emission rates and patterns in previously selected kitchens. The species is native to tropical and southern Africa but has become a widely cultivated ornamental plant in Europe.
C. comosum has proven a good candidate for phytoremediation of pollutants such as benzene (Sriprapat and Strand 2016), ethylbenzene (Sriprapat et al. 2014), toluene (Treesubsuntorn and Thiravetyan 2018), and particulate matter (Gawrońska and Bakera 2015) from indoor air. C. comosum was reported to have one of the highest VOC removal efficiency in comparison to other plant species tested (Treesubsuntorn et al. 2020). The species could take up and break down formaldehyde in the leaves (Su and Liang 2015).
Morphological characteristics make the plant a promising accumulator. Good bioaccumulator capacity is generally attributed to the following morphological parameters: leaf size/shape (plants with high surface-to-volume ratio; roughness and/or hairiness of the leaves) (Franzaring and van der Eerden 2000); presence of epicuticular wax (Li et al. 2017b). The plant has long narrow leaves (length 20–45 cm and width 6–25 millimetres). Leaves also contain a well-developed wax layer (Fermo et al. 2021). C. comosum is also a good choice for pot studies as the plant reproduces vegetatively and genetically uniform test material can be produced. The plant tolerates semi-shade, making it useable in indoor environments.
In the study of Fermo et al. (2021), C. comosum was applied to biomonitor heavy metal levels in different sites of Milan (Italy). The species has adapted to the climatic conditions of Milan, therefore outdoor air quality could be monitored with special regard of heavy metal levels such as Zn, Mn, Cd, Cr, Co, Ni, and Pb.
While a wide range of studies have addressed the magnitude and impact of emissions generated by Asian cooking (Feng et al. 2021; Lin et al. 2019), much less data are available for European domestic kitchens (e.g., Alves et al. 2021). According to pre-COVID studies, the role of eating out in Hungary has been low compared to international habits (Lehota et al. 2015) which might emphasize the importance to study human exposure to cooking-generated pollution. Hungarian cooking can also be regarded as specific in comparison to Western European style taking into consideration the relatively high share of lard (Grasgruber et al. 2018).
The main goal of our study was that in addition to gaining a comprehensive picture about PAH exposure in typical rural Hungarian kitchens, we wanted to examine and establish the applicability of C. comosum for indoor bioaccumulation studies.
Material and methods
Pot study
C. comosum ‘Variegatum’ plant was purchased from a local retainer. Plantlets of similar size were separated. They were acclimatized in uncontaminated commercial soil (pH: 6.8 ± 0.5; N (m/m%): min 0.3; P2O5 (m/m%): min 0.1; and K2O (m/m%): min 0.3) and placed in a greenhouse for four weeks before the study. In each kitchen, 4 plants were used.
Exposure started 1 June and ended 31 July. This period was chosen to avoid potential cross-pollution from heating. Normally heating season ends in April in Hungary but May was exceptionally cold in 2021, therefore several households continued heating in May. Another reason was for selection that during summer holidays children mostly lunch at home, which increases cooking frequency.
After the first month, 1-month-old leaves while after the second month, 2-month-old leaves were selected and cut with pre-washed scissors (using ultrapure water and ethanol). Leaves were immediately taken to the laboratory, washed, freeze dried, ground, homogenized (Wang et al. 2021), and kept in the freezer (− 20 °C) until analysis.
Household selection
Households with approximately the similar size were selected (2 adults + 2 children) (Table 1). Another important criterion was the distance from main roads: all households are situated in small villages, not affected by heavy traffic. This criterion was especially important as some indoor biomonitoring studies reported that in case of traffic-impacted sites such as schools, infiltration of outdoor air pollutants could be experienced (Paoli et al. 2019, Protano et al. 2017).
Determination of the PAH content
Ten-gram plant sample was grinded and extracted with 20 mL n-hexane. Ten-milliliter acetone was added prior to extraction, and 100 μL of 0.01 μg/mL deuterated PAH surrogate mixture (naphtalene-d8, acenaphthene-d10, phenanthrene-d10, chryzene-d12, benzo(a)pyrene-d12, and perylene-d12, from Restek Corporation, Bellefonte, Pennsylvania, USA) were spiked to it. Dry nitrogen stream was used to concentrate the extract to 1 mL, and an additional solid phase silica gel and alumina oxide sample clean-up was performed. 100 μL of 0.01 μg/mL internal standard (p-terphenyl-d14, 2-fluorobiphenyl from Restek Corporation, Bellefonte, Pennsylvania, USA) was added to the clean sample. The plant samples were analyzed by an HP-6890 gas chromatograph coupled to an HP-5973 (Agilent Technologies, Palo-Alto, USA) quadrupole mass spectrometer (low-resolution single MS). The standards were properly diluted with GC grade solvents (Sigma-Aldrich, St. Louis, Missouri USA) and prepared freshly before the analysis.
During the analysis the head pressure of the column was 50PSI. ZB-Semivolatiles (Phenomenex, Torrance California USA) GC column was used). For 3 minutes after injection, the temperature of the GC oven was 40 °C and heat up (40 °C/min) to 80 °C for 0.5 min.With small breaks, the temperature was increased (15 °C/min) to 310 °C. With constant flow rate (1.2 mL/min) of the helium (N55, Linde Dublin Ireland), carrier gas was used. During the callibration for each target, compound from the standard mixture was estabilished. Five different concentrations between 0.5 and 5.0 ng/mL was detected to determinate the calibration curve.
The measurement was based on MSZ EN 15527:2009 (characterization of waste, determination of polycyclic aromatic hydrocarbons (PAH) in waste using gas chromatography mass spectrometry) (more detailed description was given in Hubai et al. (2021)). The limit of PAH detection (LOD) in extract was 0.001 μg/L and in plant samples 0.1 μg/kg dry plant material.
Analytical determinations were performed by courtesy of the Laboratory of the ELGOSCAR-2000 Environmental Technology and Water Management Ltd. accredited by the National Accreditation Authority, registration number NAH-1-1278/2015.
Results and discussion
Measured PAH concentrations in the selected households
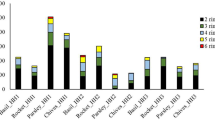
Prior to the test, PAH determination was carried out. No PAHs were detected. In HH1, concentration of total PAHs showed a clear time dependency, being 127 μg/kg at the end of the first month of the exposure and 236 μg/kg at the end of the second month. LMW PAHs showed this accumulation pattern, e.g., concentration of Acy was 1.2 μg/kg and 3.3 μg/kg, Ace was 1 μg/kg and 1.7 μg/kg, and Flu 3.2 μg/kg and 5.7 μg/kg. On the other hand, some HMW PAHs such as B(g,h,i,)p showed a marked increase in the second month of the exposure, as its concentration was 1.1 μg/kg at the end of the first month but 8.1 μg/kg at the end of the second month. Concentration of the carcinogenic B(a)p was 0 μg/kg at the first half of the exposure period but grew to 5.1 μg/kg by the end of the exposure (Fig. 1.).
In all households, the dominant PAH was Nap (HH1: 103 μg/kg and 125 μg/kg, HH2: 44 μg/kg and 260 μg/kg, and HH3: 195 μg/kg, respectively), in concordance with other studies (e.g., Zhu and Wang 2003, Sharma and Jain 2020).
In HH2, concentration of total PAHs was 133.6 μg/kg and more than tripled by the end of the second month, reaching as much as 411.5 μg/kg. The greatest differences in comparison to HH1 were the lack of certain PAHs: for example, the carcinogenic B(a)p could not be detected. No 6-ring PAHs were detected, either.
In HH2, the dramatic increase between Month1 and Month 2 can be attributed to Nap: its concentration was 44 and 260 μg/kg, respectively. Concentration of 3-ring PAHs also showed a marked increase, in case of Phen for example this increase was from 16 to 51 μg/kg. However, concentration of higher MW PAHs showed a less clear tendency: for example concentration of the 5-ring B(e)p increased from 1 to 3.3 μg/kg while concentration of B(k)f remained practically the same (4.3 and 4.4 μg/kg) (Fig. 2).
In HH3, results are given only for the first month’s exposure, due to the damage of the test system (Fig. 3). Total amount of PAHs, however, reached as much as 471.18 μg/kg. The second dominant PAH was Phen (124 μg/kg). Concentration of the carcinogenic B(a)p was also relatively high, 6.7 μg/kg.
In HH4 (Fig. 4), total amount of accumulated PAHs was lower than in the other sampling sites, 146 and 224.5 μg/kg by the end of the two exposure periods. Similarly to HH3, the second dominant PAH was Phen (37 and 45 μg/kg, respectively). Concentrations of the carcinogenic B(a)p were rather similar comparing Month1 and Month2, 1.9 and 2.3 μg/kg, respectively. Interestingly, concentration of B(e)p increased from 1.1 to 8.3 μg/kg by the end of the study.
Phen was found at high concentrations in the study of Sun et al. (2020), when different Chinese cooking styles were compared. Phen was characteristic of Sichuan cuisine, which is mixed with quick frying, high-temperature cooking, and large oil consumption. In residential Chinese kitchens, Phen was one of the most characteristic PAHs in the emissions from water-based cooking activities (Zhao et al. 2019). In general, the relatively high share of Phen in HH2–HH4 might raise some human health concern as in the study of Shin et al. (2013) where the main exposure pathway to Nap and Phen was inhalation from indoor sources in developed countries.
See et al. (2006) reported that in general, frying operations such as stir- and deep-frying generated higher molecular weight compounds. These operations are very characteristic of Asian cooking: extremely high amount of IP was reported by Singh et al. (2016) in samples taken in a North Indian commercial kitchen. Zhang et al. (2017) also detected high concentrations of B(a)P, B(b)F, B(a)A, IP, and Cry during domestic Chinese cooking. It might explain the relatively high share of these PAHs in HH1, as deep-frying accounted for approximately 30% of cooking operations in this kitchen. Li et al. (2018) report that in general, cooking oil fumes contain such harmful PAHs as B(a)P, B(b)F, B(a)A, and DBA. The carcinogenic B(a)P was found a significant fraction of particle-bound PAHs in different oils in the study of Chiang et al. (2022) when deep-frying emissions were analyzed. Lin et al. (2022) suggest that cooking oil is the major source of PAH emissions instead of the food being cooked.
HMW PAHs were also found in university canteens and a charcoal-grilled chicken restaurant in Portugal (Vicente et al. 2021). Alves et al. (2021) compared emission of particulate matter (PM10, PM2.5, and PM1) and total volatile organic compounds (TVOCs) during cooking different typical Latin meals such as stuffed chicken, fried mackerel, and fried and grilled pork. In general, emissions from grilled pork contained PAHs in the highest concentration, including HMW PAHs such as IP, D(a,h)a, and B(g,h,i,)p.
Hu et al. (2021) studied the behaviour of PAHs during deep frying with special regard to the mutagenic B(a)P and found that PAH level in sunflower oil generally accumulated with increasing frying time. It might explain the drastic increase of this PAH in HH1, too. In general, B(a)P emission was found characteristic during deep frying in the study of Yao et al. (2015). In our study, it was detected in households using this cooking method such as HH1, HH3, and HH4.
HH1 was also the only household where olive oil was used at relatively high frequency (approximately 5%). Wang et al. (2018) compared particle emission characteristics originated from using four types of oil (soybean oil, olive oil, peanut oil, and lard) and found olive oil emitting the highest number of particles.
Accumulation capacity of Chlorophytum comosum
Several studies have reported the relatively higher accumulation rate of LMW PAHs (Jia et al. 2018, Wang et al. 2017). Exposure pathways do differ to some extent: more volatile LMW PAHs are available in gas phase, while HMW PAHs are less volatile and occur mostly in particulates (Mukhopadhyay et al. 2020).
On the other hand, accumulation pattern might highly depend on the taxon used (Huang et al. 2018). Capozzi et al. (2017) found that Robinia pseudacacia leaves were able to accumulate both LMW and HMW PAHs in a field study. Similar bioaccumulation capacity was reported for the perennial Plantago lanceolata (Bakker et al. 1999, Hubai et al. 2021). Relatively high share of 5-ring and 6-ring PAHs was found in Poaceae species such as rice (Tao et al. 2006) and grass (Borgulat and Staszewski 2018). Positive correlation was found between atmospheric and accumulated PAH concentrations in maize (Lin et al. 2007).
Figure 5 shows the amount of PAH isomers in the different Chlorophytum samples. The relatively high share of 5-ring and 6-ring PAHs in HH1 at the end of the total exposure (33.9 μg/kg and 15.1 μg/kg) clearly shows that this species is able to accumulate HMW PAHs. Five-ring PAHs also reach considerable concentrations in Chlorophytum leaves in HH2 and HH4 (13 μg/kg and 19.8 μg/kg by the end of the second month, respectively) and 33.6 in HH3 by the end of the first month of exposure.
The formation of HMW PAHs is generally associated with high-temperature cooking such as deep-frying which is done at 170–220 °C (Li et al. 2022). Zhang et al. (2009) reported that these PAHs might also be associated with frying in sunflower oil. Of HMW PAHs, IP was found to be a potential marker for deep-frying which had a high share in HH1 (Xu et al. 2020).
Conclusions
Accumulation of PAHs was assessed in 4 Hungarian kitchens over 1- and 2-month-long exposures. The selected kitchens shared important characteristic features such as size of the household, but significantly differed considering materials used and cooking practices. Most important difference was the use of sunflower oil and deep-frying as a cooking method. In these households, the relative share of HMW PAHs was considerably higher than in the household which used lard and butter. Deep-frying was also indicated by detectable amounts of the carcinogenic B(a)p. Nevertheless, the dominant PAH was Nap, in concordance with reported studies. PAH accumulation showed a clear time-dependent pattern, as concentrations were considerably higher at the end of the total exposure period (2 months) than after 1-month exposure. C. comosum was able to accumulate both LMW and HMW PAHs, so it has proven a sensitive biomonitor for indoor PAH levels. Its use as biomonitor is further increased by the popularity and easy-to-cultivate nature of this ornamental plant.
Abbreviations
- PM:
-
Particulate matter
- PAH:
-
Polycyclic aromatic hydrocarbon
- LMW:
-
Low molecular weight
- HMW:
-
High molecular weight
- Nap:
-
Naphthalene
- Acy:
-
Acenaphthylene
- Ace:
-
Acenaphthene
- Flu:
-
Fluorene
- Phen:
-
Phenanthrene
- Ant:
-
Anthracene
- Flt:
-
Fluoranthene
- Pyr:
-
Pyrene
- B(a)a:
-
Benzo[a]anthracene
- Cry:
-
Chrysene
- B(b)f:
-
Benzo[b]fluoranthene
- B(k)f:
-
Benzo[k]fluoranthene
- B(e)p:
-
Benzo[e]pyrene
- B(a)p:
-
Benzo[a]pyrene
- IP:
-
Indeno[1,2,3-cd]pyrene
- D(a,h)a:
-
Dibenzo[a,h]anthracene
- B(g,h,i)p:
-
Benzo[g,h,i]perylene
References
Alves CA, Vicente ED, Evtyugina M, Vicente AMP, Sainnokhoi TA, Kováts N (2021) Cooking activities in a domestic kitchen: chemical and toxicological profiling of emissions. Sci Total Environ 772:145412. https://doi.org/10.1016/j.scitotenv.2021.145412
Bakker MI, Vorenhout M, Sijm DT, Kollöffel C (1999) Dry deposition of atmospheric polycyclic aromatic hydrocarbons in three Plantago species. Environ Toxicol Chem 18:2289–2294. https://doi.org/10.1002/etc.5620181025
Baldantoni D, Alfani A (2016) Usefulness of different vascular plant species for passive biomonitoring of Mediterranean rivers. Environ Sci Pollut Res 23:13907–13917. https://doi.org/10.1007/s11356-016-6592-6
Borgulat J, Staszewski T (2018) Fate of PAHs in the vicinity of aluminum smelter. Environ Sci Pollut Res 25:26103–26113. https://doi.org/10.1007/s11356-018-2648-0
Canha N, Almeida-Silva M, Freitas MC, Almeida SM, Wolterbeek HT (2012) Lichens as biomonitors at indoor environments of primary schools. J Radioanal Nucl Chem 291:123–128. https://doi.org/10.1007/s10967-011-1259-8
Capozzi F, Di Palma A, Adamo P, Spagnuolo V, Giordano S (2017) Monitoring chronic and acute PAH atmospheric pollution using transplants of the moss Hypnum cupressiforme and Robinia pseudacacia leaves. Atmos Environ 150:45–54. https://doi.org/10.1016/j.atmosenv.2016.11.046
Chen Y, Ho KF, Ho SSH, Ho WK, Lee SC et al (2007) Gaseous and particulate polycyclic aromatic hydrocarbons (PAHs) emissions from commercial restaurants in Hong Kong. J Environ Monit 9:1402–1409. https://doi.org/10.1039/b710259c
Chiang KM, Xiu L, Peng CY, Lung SCC, Chen YC, Pan WH (2022) Particulate matters, aldehydes, and polycyclic aromatic hydrocarbons produced from deep-frying emissions: comparisons of three cooking oils with distinct fatty acid profiles. Sci Food 6:28; doi:https://doi.org/10.1038/s41538-022-00143-5
Cho H, Youn JS, Oh I, Jung YW, Jeon KJ (2019) Determination of the emission rate for ultrafine and accumulation mode particles as a function of time during the pan-frying of fish. J Environ Manage 236:75–80. https://doi.org/10.1016/j.jenvman.2018.12.010
Feng S, Shen X, Hao X et al (2021) Polycyclic and nitro-polycyclic aromatic hydrocarbon pollution characteristics and carcinogenic risk assessment of indoor kitchen air during cooking periods in rural households in North China. Environ Sci Pollut Res 28:11498–11508. https://doi.org/10.1007/s11356-020-11316-8
Fermo P, Masiero S, Rosa M, Labella G, Comite V (2021) Chlorophytum comosum: a bio-indicator for assessing the accumulation of heavy metals present in the aerosol particulate matter (PM). Appl Sci 11:4348. https://doi.org/10.3390/app11104348
Franzaring J, van der Eerden LJM (2000) Accumulation of airborne persistent organic pollutants (POPs) in plants. Basic Appl Ecol 1:25–30. https://doi.org/10.1078/1439-1791-00003
Gawrońska H, Bakera B (2015) Phytoremediation of particulate matter from indoor air by Chlorophytum comosum L. plants. Air Qual Atmos Health 8:265–272. https://doi.org/10.1007/s11869-014-0285-4
González-Martín J, Kraakman NJR, Pérez C, Lebrero R, Muñoz R (2021) A state-of-the-art review on indoor air pollution and strategies for indoor air pollution control. Chemosphere. 262:128376. https://doi.org/10.1016/j.chemosphere.2020.128376
Grasgruber P, Hrazdira E, Sebera M, Kalina T (2018) Cancer incidence in Europe: an ecological analysis of nutritional and other environmental factors. Front. Oncol. 8:151. https://doi.org/10.3389/fonc.2018.00151
Hao X, Li J, Yao Z (2016) Changes in PAHs levels in edible oils during deep-frying process. Food Control 66:233–240. https://doi.org/10.1016/j.foodcont.2016.02.012
Hu M, Zhu M, Xin L, Zhang G, Wu S, Hu X, Gong D (2021) Change of benzo(a)pyrene during frying and its groove binding to calf thymus DNA. Food Chem 350:129276. https://doi.org/10.1016/j.foodchem.2021.129276
Huang S, Dai C, Zhou Y, Peng H, Yi K, Qin P, Luo S, Zhang X (2018) Comparisons of three plant species in accumulating polycyclic aromatic hydrocarbons (PAHs) from the atmosphere: a review. Environ Sci Pollut Res 25:16548–16566. https://doi.org/10.1007/s11356-018-2167-z
Huang L, Cheng H, Ma S, He R, Gong J, Li G, An T (2021) The exposures and health effects of benzene, toluene and Nap for Chinese chefs in multiple cooking styles of kitchens. Environ Int 156:106721. https://doi.org/10.1016/j.envint.2021.106721
Hubai K, Kováts N, Sainnokhoi TA, Teke G (2021) Accumulation pattern of polycyclic aromatic hydrocarbons using Plantago lanceolata L. as passive biomonitor. Environ Sci Pollut Res 199. https://doi.org/10.1007/s11356-021-16141-1
IARC (2010) IARC monographs on the evaluation of carcinogenic risks to humans.some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. International Agency for Research on Cancer, Lyon, France
Iwegbue CMA, Osijaye KO, Igbuku UA, Egobueze FE, Tesi GO, Bassey FI, Martincigh BS (2020) Effect of the number of frying cycles on the composition, concentrations and risk of polycyclic aromatic hydrocarbons (PAHs) in vegetable oils and fried fish. J Food Compost Anal 94:103633. https://doi.org/10.1016/j.jfca.2020.103633
Jia J, Bi C, Zhang J, Jin X, Chen Z (2018) Characterization of polycyclic aromatic hydrocarbons (PAHs) in vegetables near industrial areas of Shanghai, China: Sources, exposure, and cancer risk. Environ Pollut 241:750–758. https://doi.org/10.1016/j.envpol.2018.06.002
Juániz I, Zocco C, Mouro V, Cid C, De Pena MP (2016) Effect of frying process on furan content in foods and assessment of furan exposure of Spanish population. LWT - Food Sci Technol 68:549–555. https://doi.org/10.1016/j.lwt.2015.12.061
Lehota J, Könyves E, Dunay A (2015) Key factors of a successful restaurant strategy in Hungary. In: Dunnay A (ed) Proceedings of the 5th International Conference on Management. Szent István University Publishing House, Gödöllő, pp 22–27. https://doi.org/10.17626/dBEM.ICoM.P00.2015.p005
Li J (2021) Insights into cooking sources in the context of sustainable development goals. Sustain Prod Consum 26:517–531. https://doi.org/10.1016/j.spc.2020.12.017
Li Z, Sjödin A, Romanoff LC, Horton K, Fitzgerald CL, Eppler A, Aguilar-Villalobos M, Naeher LP (2011) Evaluation of exposure reduction to indoor air pollution in stove intervention projects in Peru by urinary biomonitoring of polycyclic aromatic hydrocarbon metabolites. Environ Int 37:1157–1163. https://doi.org/10.1016/j.envint.2011.03.024
Li S, Gao J, He Y, Cao L, Li A, Mo S, Chen Y, Cao Y (2017a) Determination of time- and size-dependent fine particle emission with varied oil heating in an experimental kitchen. J Environ Sci (China) 51:157–164. https://doi.org/10.1016/j.jes.2016.06.030
Li Q, Li Y, Zhu L, Xing B, Chen B (2017b) Dependence of plant uptake and diffusion of polycyclic aromatic hydrocarbons on the leaf surface morphology and micro-structures of cuticular waxes. Sci rep 7:46235. https://doi.org/10.1038/srep46235
Li YC, Qiu JQ, Shu M, Ho SSH, Cao JJ et al (2018) Characteristics of polycyclic aromatic hydrocarbons in PM2.5 emitted from different cooking activities in China. Environ Sci Pollut Res 25:4750–4760. https://doi.org/10.1007/s11356-017-0603-0
Li L, Cheng Y, Dai Q, Liu B, Wu J et al (2022) Chemical characterization and health risk assessment of VOCs and PM2.5-bound PAHs emitted from typical Chinese residential cooking. Atmos Environ 291:119392. https://doi.org/10.1016/j.atmosenv.2022.119392
Lin H, Tao S, Zuo Q, Coveney RM (2007) Uptake of polycyclic aromatic hydrocarbons by maize plants. Environ Pollut 148:614–619. https://doi.org/10.1016/j.envpol.2006.11.026
Lin P, He W, Nie L et al (2019) Comparison of PM2.5 emission rates and source profiles for traditional Chinese cooking styles. Environ Sci Pollut Res 26:21239–21252. https://doi.org/10.1007/s11356-019-05193-z
Lin C, Huang RJ, Duan J, Zhong H, Xu W (2022) Polycyclic aromatic hydrocarbons from cooking emissions. Sci Total Environ 818:151700. https://doi.org/10.1016/j.scitotenv.2021.151700
Liu Y, Li J, Cheng Y, Liu Y (2019) Effect of frying oils’ fatty acid profile on quality, free radical and volatiles over deep-frying process: a comparative study using chemometrics. LWT - Food Sci Technol 101:331–341. https://doi.org/10.1016/j.lwt.2018.11.033
Mukhopadhyay S, Dutta R, Das P (2020) A critical review on plant biomonitors for determination of polycyclic aromatic hydrocarbons (PAHs) in air through solvent extraction techniques. Chemosphere 251:126441. https://doi.org/10.1016/j.chemosphere.2020.126441
Murawski A, Roth A, Schwedler G, Schmied-Tobies MIH, Rucic E, Pluym N, Scherer M, Scherer G, Conrad A, Kolossa-Gehring M (2020) Polycyclic aromatic hydrocarbons (PAH) in urine of children and adolescents in Germany – human biomonitoring results of the German Environmental Survey 2014–2017 (GerES V). Int J Hyg Environ Health 226:113491. https://doi.org/10.1016/j.ijheh.2020.113491
Neupane B, Kang S, Li C, Chen P (2019) Trace elements analysis in hair strand of cooks chronically exposed to indoor air pollution in restaurants of Lhasa, Tibet: preliminary results. SN Appl Sci 1:1039. https://doi.org/10.1007/s42452-019-0890-9
Ng CY, Leong XF, Masbah N, Adam SK, Kamisah Y, Jaarin K (2014) Heated vegetable oils and cardiovascular disease risk factors. Vasc Pharmacol 61:1–9. https://doi.org/10.1016/j.vph.2014.02.004
Oliveira M, Slezakova K, Delerue-Matos C, Pereira MC, Morais S (2017) Assessment of exposure to polycyclic aromatic hydrocarbons inpreschool children: levels and impact of preschool indoor air onexcretion of main urinary monohydroxyl metabolites. J Hazard Mater 322:357–369. https://doi.org/10.1016/j.jhazmat.2016.10.004
Paoli L, Fačkovcová Z, Guttová A, Maccelli C, Kresáńová K, Loppi SE (2019) Goes to school: bioaccumulation of heavy metals and photosynthetic performance in lichen transplants exposed indoors and outdoors in public and private environments. Plants 8:125. https://doi.org/10.3390/plants8050125
Preuss R, Angerer J, Drexler H (2003) Naphthalene—an environmental and occupational toxicant. Int Arch Occup Environ Health 76:556–576. https://doi.org/10.1007/s00420-003-0458-1
Protano C, Owczarek M, Antonucci A, Guidotti M, Vitali M (2017) Assessing indoor air quality of school environments: transplanted lichen Pseudovernia furfuracea as a new tool for biomonitoring and bioaccumulation. Environ Monit Assess 189:358. https://doi.org/10.1007/s10661-017-6076-2
Rutkowski R, Rybak J, Rogula-Kozłowska W, Bełcika M, Piekarska K, Jureczko I (2019) Mutagenicity of indoor air pollutants adsorbed on spider webs. Ecotoxicol Environ 171:549–557. https://doi.org/10.1016/j.ecoenv.2019.01.0191
Rybak J, Rogula-Kozłowska W, Jureczko I, Rutkowski R (2019) Monitoring of indoor polycyclic aromatic hydrocarbons using spider webs. Chemosphere 218:758–766. https://doi.org/10.1016/j.chemosphere.2018.11.174
See SW, Balasubramanian R (2006) Risk assessment of exposure to indoor aerosols associated with Chinese cooking. Environ Res 102:197–204. https://doi.org/10.1016/j.envres.2005.12.013
See SW, Karthikeyan S, Balasubramanian R (2006) Health risk assessment of occupational exposure to particulate-phase polycyclic aromatic hydrocarbons associated with Chinese, Malay and Indian cooking. J Environ Monit 8:369–376. https://doi.org/10.1039/B516173H
Sharma D, Jain S (2020) Carcinogenic risk from exposure to PM2.5 bound polycyclic aromatic hydrocarbons in rural settings. Ecotoxicol Environ Saf 190:110135. https://doi.org/10.1016/j.ecoenv.2019.110135
Shin HM, McKone TE, Bennett DH (2013) Evaluating environmental modeling and sampling data with biomarker data to identify sources and routes of exposure. Atmos Environ 69:148–155. https://doi.org/10.1016/j.atmosenv.2012.12.027
Singh A, Nair KC, Kamal R, Bihari V, Gupta MK, Mudiam MKR, Satyanarayana GNV, Raj A, Haq I, Shukla NK, Khan AH, Srivastava AK (2016) Assessing hazardous risks of indoor airborne polycyclic aromatic hydrocarbons in the kitchen and its association with lung functions and urinary PAH metabolites in kitchen workers. Clin Chim Acta 452:204–213. https://doi.org/10.1016/j.cca.2015.11.020
Sjaastad AK, Svendsen K (2009) Exposure to polycyclic aromatic hydrocarbons(PAHs), mutagenic aldehydes, and particulate matter in Norwegian à la Carte restaurants. Ann Occup Hyg 53:723–729. https://doi.org/10.1093/annhyg/mep059
Sriprapat W, Strand SE (2016) A lack of consensus in the literature findings on the removal of airborne benzene by houseplants: effect of bacterial enrichment. Atmos Environ 131:9–16. https://doi.org/10.1016/j.atmosenv.2016.01.031
Sriprapat W, Suksabye P, Areephak S, Klantup P, Waraha A, Sawattan TP (2014) Uptake of toluene and ethylbenzene by plants: removal of volatile indoor air contaminants. Ecotoxicol Environ Saf 102:147–151. https://doi.org/10.1016/j.ecoenv.2014.01.032
Su Y, Liang Y (2015) Foliar uptake and translocation of formaldehyde with bracket plants (Chlorophytum comosum). J Hazard Mater 291:120–128. https://doi.org/10.1016/j.jhazmat.2015.03.001
Sun J, Shen Z, Niu X, Yu J, Zhang Y, Liu S, Niu X, Zhang Y, Xu H, Li X, Cao J (2020) PM2.5 source profiles from typical Chinese commercial cooking activities in northern China and its influences on bioreactivity of vascular smooth muscle cells (VSMCs). Atmos Environ 239:117750. https://doi.org/10.1016/j.atmosenv.2020.117750
Tao S, Jiao XC, Chen SH, Liu WX, Coveney RM, Zhu LZ, Luo YM (2006) Accumulation and distribution of polycyclic aromatic hydrocarbons in rice (Oryza sativa). Environ Pollut 140:406–415. https://doi.org/10.1016/j.envpol.2005.08.004
Torkmahalleh MA, Gorjinezhad S, Unluevcek HS, Hopke PK (2017) Review of factors impacting emission/concentration of cooking generated particulate matter. Sci Total Environ 586:1046–1056. https://doi.org/10.1016/j.scitotenv.2017.02.088
Touffet M, Trystram G, Vitrac O (2020) Revisiting the mechanisms of oil uptake duringdeep-frying. Food Bioprod Process 123:14–30. https://doi.org/10.1016/j.fbp.2020.06.007
Treesubsuntorn C, Thiravetyan P (2018) Botanical biofilter for indoor toluene removal and reduction of carbon dioxide emission under low light intensity by using mixed C3 and CAM plants. J Clean Prod 194:94–100. https://doi.org/10.1016/j.jclepro.2018.05.141
Treesubsuntorn C, Lakaew K, Autarmat S, Thiravetyan P (2020) Enhancing benzene removal by Chlorophytum comosum under simulation microgravity system: effect of light-dark conditions and indole-3-acetic acid. Acta Astronaut 175:396–404. https://doi.org/10.1016/j.actaastro.2020.05.061
van Laaten N, Merten D, von Tümpling W, Schäfer T, Pirrung M (2020) Comparison of spider web and moss bag biomonitoring to detect sources of airborne trace elements. Water Air Soil Pollut 231:512. https://doi.org/10.1007/s11270-020-04881-8
Vicente AMP, Rocha S, Duarte M, Moreira R, Nunes T, Alves CA (2021) Fingerprinting and emission rates of particulate organic compounds from typical restaurants in Portugal. Sci Total Environ 778:146090. https://doi.org/10.1016/j.scitotenv.2021.146090
Wallace L, Ott W (2011) Personal exposure to ultrafine particles. J Expo Sci Environ Epidemiol 21:20–30. https://doi.org/10.1038/jes.2009.59
Wang G, Cheng S, Wei W, Wen W, Wang X, Yao S (2015) Chemical characteristics of fine particles emitted from different Chinese cooking styles. Aerosol Air Qual Res 15:2357–2366. https://doi.org/10.4209/aaqr.2015.02.0079
Wang J, Zhang X, Ling W, Liu R, Liu J, Kang F, Gao S (2017) Contamination and health risk assessment of PAHs in soils and crops in industrial areas of the Yangtze River Delta region. China, Chemosphere 168:976–987. https://doi.org/10.1016/j.chemosphere.2016.10.113
Wang L, Zheng X, Stevanovic S, Wu X, Xiang Z, Yu M, Liu J (2018) Characterization particulate matter from several Chinese cooking dishes and implications in health effects. J Environ Sci (China) 72:98–106. https://doi.org/10.1016/j.jes.2017.12.015
Wang Y, Zhang Z, Tan F, Rodgers TFM, Hou M et al (2021) Ornamental houseplants as potential biosamplers for indoor pollution of organophosphorus flame retardants. Sci Total Environ 767:144433. https://doi.org/10.1016/j.scitotenv.2020.144433
Xu H, Ta W, Yang L, Feng R, He K et al (2020) Characterizations of PM2.5-bound organic compounds and associated potential cancer risks on cooking emissions from dominated types of commercial restaurants in northwestern China. Chemosphere 261:127758. https://doi.org/10.1016/j.chemosphere.2020.127758
Yao Z, Li J, Wu B, Hao X, Yin Y, Jiang X (2015) Characteristics of PAHs from deep-frying and frying cooking fumes. Environ Sci Pollut Res 22:16110–16120. https://doi.org/10.1007/s11356-015-4837-4
Zhai SR, Albritton D (2020) Airborne particles from cooking oils: emission test and analysis on chemical and health implications. Sustain Cities Soc 52:101845. https://doi.org/10.1016/j.scs.2019.101845
Zhang L, Bai Z, You Y, Wu J, Feng Y, Tan Zhu T (2009) Chemical and stable carbon isotopic characterization for PAHs in aerosol emitted from two indoor sources. Chemosphere 75:453–461. https://doi.org/10.1016/j.chemosphere.2008.12.063
Zhang N, Han B, He F, Xu J, Zhao R, Zhang RY, Bai Z (2017) Chemical characteristic of PM2.5 emission and inhalational carcinogenic risk of domestic Chinese cooking. Environ Pollut 227:24–30. https://doi.org/10.1016/j.envpol.2017.04.033
Zhao X, He M, Shang H, Yu H, Wang H et al (2018) Biomonitoring polycyclic aromatic hydrocarbons by Salix matsudana leaves: a comparison with the relevant air content and evaluation of environmental parameter effects. Atmos Environ 181:47–53. https://doi.org/10.1016/j.atmosenv.2018.03.004
Zhao Y, Chen C, Zhao B (2019) Emission characteristics of PM2.5-bound chemicals from residential Chinese cooking. Build Environ 149:623–629. https://doi.org/10.1016/j.buildenv.2018.12.060
Zhu L, Wang J (2003) Sources and patterns of polycyclic aromatic hydrocarbons pollution in kitchen air, China. Chemosphere 50:611–618. https://doi.org/10.1016/s0045-6535(02)00668-9
Acknowledgements
The authors thank the ELGOSCAR-2000 Environmental Technology and Water Management Ltd. (Head Office: 164 Soroksari u. H-1095 Budapest, Laboratory: H-8184 Balatonfuzfo) for conducting analytical measurements.
Data availability
All data generated or analyzed during this study are included in this published article.
Funding
Open access funding provided by University of Pannonia. Financial support was provided by the NKFIH-872 project ‘Establishment of a National Multidisciplinary Laboratory for Climate Change.’
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Conceptualization was performed by KH. Data collection and analysis were performed by KH, NK, BEV, and GT. The first draft of the manuscript was written by NK and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This is not relevant.
Consent for publication
This is not relevant.
Competing interests
The authors declare no conflicts of interest.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hubai, K., Kováts, N., Eck-Varanka, B. et al. Pot study using Chlorophytum comosum plants to biomonitor PAH levels in domestic kitchens. Environ Sci Pollut Res 30, 51932–51941 (2023). https://doi.org/10.1007/s11356-023-25469-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-25469-9