Abstract
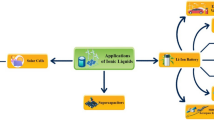
Ionic liquids (ILs), often known as green designer solvents, have demonstrated immense application potential in numerous scientific and technological domains. ILs possess high boiling point and low volatility that make them suitable environmentally benign candidates for many potential applications. The more important aspect associated with ILs is that their physicochemical properties can be effectively changed for desired applications just by tuning the structure of the cationic and/or anionic part of ILs. Furthermore, these eco-friendly designer materials can function as electrolytes or solvents depending on the application. Owing to the distinctive properties such as low volatility, high thermal and electrochemical stability, and better ionic conductivity, ILs are nowadays immensely used in a variety of energy applications, particularly in the development of green and sustainable energy storage and conversion devices. Suitable ILs are designed for specific purposes to be used as electrolytes and/or solvents for fuel cells, lithium-ion batteries, supercapacitors (SCs), and solar cells. Herein, we have highlighted the utilization of ILs as unique green designer materials in Li-batteries, fuel cells, SCs, and solar cells. This review will enlighten the promising prospects of these unique, environmentally sustainable materials for next-generation green energy conversion and storage devices. Ionic liquids have much to offer in the field of energy sciences regarding fixing some of the world’s most serious issues. However, most of the discoveries discussed in this review article are still at the laboratory research scale for further development. This review article will inspire researchers and readers about how ILs can be effectively applied in energy sectors for various applications as mentioned above.










Similar content being viewed by others
Data availability
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Abbreviations
- HDDA:
-
1,6-Hexanediol diacrylate
- AMII:
-
1–Allyl–3–methylimidazolium iodide
- BMIM Cl:
-
1-Butyl-3-methylimidazolium chloride
- BMII:
-
1-Butyl-3-methylimidazolium iodide
- BMIMBF4 :
-
1-Butyl-3-methylimidazolium tetrafluoroborate
- EMITFSI:
-
1-Ethyl-3-methylimidazolium bis(trifuoromethylsulfonyl)imide
- EMIm:
-
1-Ethyl-3-methylimidazolium
- EMIM:
-
1-Ethyl-3-methylimidazolium
- EMIM Ac:
-
1-Ethyl-3-methylimidazolium acetate
- [EMIm][Tf2N]:
-
1-Ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide
- EMIm TFSI:
-
1-Ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide
- EMImDCN:
-
1-Ethyl-3-methylimidazolium dicyanamide
- EMIBF4 :
-
1-Ethyl-3-methylimidazolium tetrafluoroborate
- HMII:
-
1-Hexyl-3-methylimidazolium iodide
- [HMIM]I:
-
1-Methyl-3-hexylimidazolium iodide
- PMII:
-
1-Methyl-3-propyl imidazolium iodide
- PMImI:
-
1-Methyl-3-propyl imidazolium iodide
- PMImDP:
-
1-Propyl-3-methylimidazolium dihydrogenphosphate
- [EMIm] [TFSI]:
-
1-Propyl-3-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide
- VHpII:
-
1-Vinyl-3-heptylimidazolium iodide
- BCP:
-
Bathocuproine
- FSI:
-
Bis(fluorosulfonyl) imide
- B.P.:
-
Boiling point
- DSSCs:
-
Dye-sensitized solar cells
- EES:
-
Electrical energy storage
- EDLC:
-
Electrochemical double-layer capacitance
- ESPWs:
-
Electrochemical stability potential windows
- ETL:
-
Electron transport layer
- ETM:
-
Electron transport materials
- FF:
-
Fill factor
- FA:
-
Formamidinium
- GO:
-
Graphene oxide
- GuNCS:
-
Guanidinium thiocyanate
- IoT:
-
Internet of Things
- ILs:
-
Ionic liquids
- M.P.:
-
Melting point
- MAF:
-
Methylammonium formate
- MAPbI3 :
-
Methylammonium lead triiodide
- MA:
-
Methylammonium
- MWNT:
-
Multiwalled carbon nanotube
- NPs:
-
Nanoparticles
- [PyrI4][NTf2]:
-
N-Butyl-N-methyl-pyrrolidinium bis(trifluoromethanesulfonyl)imide
- NMB:
-
N-Methylbenzimidazole
- VOC :
-
Open-circuit voltage
- P–HI:
-
P[((3–(4–vinylpyridine) propanesulfonic acid) iodide)–co–(acrylonitrile)]
- PCE:
-
Photon conversion efficiency
- PVDF-HFP:
-
Poly(vinylidene fluoride-co-hexafluoropropylene)
- PEG:
-
Polyethylene glycol
- PPDD:
-
Polypyridyl pendant poly(amidoamine) dendritic derivative
- PTh:
-
Polythiophene
- PIP:
-
Pyrrolidinium (PYR) and piperidinium
- JSC :
-
Short-circuit current
- SS-IL:
-
Solid-state ionic liquids
- SCs:
-
Supercapacitors
- TiOPc:
-
Titanium phthalocyanine
- ZrP:
-
Zirconium phosphate
References
Ahmad T, Madonski R, Zhang D, Huang C, Mujeeb A (2022) Data-driven probabilistic machine learning in sustainable smart energy/smart energy systems: key developments, challenges, and future research opportunities in the context of smart grid paradigm. Renew Sustain Energy Rev 160:112128
Aili D, Henkensmeier D, Martin S, Singh B, Hu Y, Jensen JO, Li Q (2020) Polybenzimidazole-based high-temperature polymer electrolyte membrane fuel cells: new insights and recent progress. Electrochem Energ Rev 3(4):793–845
Akin S, Akman E, Sonmezoglu S (2020) FAPbI3-Based perovskite solar cells employing hexyl-based ionic liquid with an efficiency over 20% and excellent long-term stability. Adv Funct Mater 30:1–8
Arico AS, Bruce P, Scrosati B, Tarascon JM, Van Schalkwijk W (2005) Nanostructured materials for advanced energy conversion and storage devices. Nat Mater 4(5):366–377
Armand M, Endres F, MacFarlane DR, Ohno H, Scrosati B (2009) Ionic-liquid materials for the electrochemical challenges of the future. Nat Mater 8:621–629
Asensio JA, Sánchez EM, Gomez-Romero P (2010) Proton-conducting membranes based on benzimidazole polymers for high-temperature PEM fuel cells. A chemical quest. Chem Soc Rev 39(8):3210–3239
Bai S, Da P, Li C, Wang Z, Yuan Z, Fu F, Kawecki M, Liu X, Sakai N, Wang JTW, Huettner S, Buecheler S, Fahlman M, Gao F, Snaith HJ (2019) Planar perovskite solar cells with long-term stability using ionic liquid additives. Nature 571(245):250
Behera K, Malek NI, Pandey S (2009) Visual evidence for formation of water-in-ionic liquid microemulsions. ChemPhysChem 10:3204–3208
Behera K, Pandey S, Kadyan A, Pandey S (2015) Ionic liquid-based optical and electrochemical carbon dioxide sensors. Sensors (Switzerland) 15:30487–30503
Bilgen S (2014) Structure and environmental impact of global energy consumption. Renewable and Sustainable Energy Reviews 38:890–902
Borup R, Meyers J, Pivovar B, Kim Y S, Mukundan, R, Garland N, ... Iwashita N (2007) Scientific aspects of polymer electrolyte fuel cell durability and degradation. Chem Rev 107(10):3904–3951
Boschloo G, Häggman L, Hagfeldt A (2006) Quantification of the effect of 4-tert-butylpyridine addition to I -/I3- redox electrolytes in dye-sensitized nanostructured TiO2 solar cells. J Phys Chem B 110:13144–13150
Brandt A, Pohlmann S, Varzi A, Balducci A, Passerini S (2013) Ionic liquids in supercapacitors. MRS Bull 38:554–559
Chandan A, Hattenberger M, El-Kharouf A, Du S, Dhir A, Self,V, ... Bujalski W (2013) High temperature (HT) polymer electrolyte membrane fuel cells (PEMFC)–a review. J Power Sources 231:264–278
Chao L, Niu T, Xia Y, Chen Y, Huang W (2021) Ionic liquid for perovskite solar cells: an emerging solvent engineering technology. Acc Mater Res 2(11):1059–1070
Che Q, Fan H, Duan X, Feng F, Mao W, Han X (2018) Layer by layer self-assembly fabrication of high temperature proton exchange membrane based on ionic liquids and polymers. J Mol Liq 269:666–674
Cheng XB, Zhang R, Zhao CZ, Zhang Q (2017) Toward safe lithium metal anode in rechargeable batteries: a review. Chem Rev 117(15):10403–10473
Chia SR, Chew KW, Show PL, Yap YJ, Ong HC, Ling TC, Chang JS (2018) Analysis of economic and environmental aspects of microalgae biorefinery for biofuels production: a review. Biotechnol J 13(6):1700618
Chmiola J, Yushin G, Gogotsi Y, Portet C, Simon P (2006) P. L. T. News of the week new ‘ supercapacitor ’ promises to pack more electrical punch. Science 313:902
Correia DM, Fernandes LC, Fernandes MM, Hermenegildo B, Meira RM, Ribeiro C, Ribeiro S, Reguera J, Lanceros-Méndez S (2021) Ionic liquid-based materials for biomedical applications. Nanomaterials 11:2401
Cullen DA, Neyerlin KC, Ahluwalia RK, Mukundan R, More KL, Borup RL, ... Kusoglu A (2021) New roads and challenges for fuel cells in heavy-duty transportation. Nat Energy 6(5):462–474
Dai Q, Menzies DB, MacFarlane DR, Batten SR, Forsyth S, Spiccia L, Cheng YB, Forsyth M (2006) Dye-sensitized nanocrystalline solar cells incorporating ethylmethylimidazolium-based ionic liquid electrolytes. Comptes Rendus Chim 9:617–621
Devrim Y, Arıca ED (2019) Multi-walled carbon nanotubes decorated by platinum catalyst for high temperature PEM fuel cell. Int J Hydrog Energy 44(34):18951–18966
Di Noto V, Lavina S, Giffin GA, Negro E, Scrosati B (2011) Polymer electrolytes: present, past and future. Electrochim Acta 57:4–13
Dicks AL, Rand DA (2018) Fuel cell systems explained. Wiley
Ding JF, Xu R, Yan C, Li BQ, Yuan H, Huang JQ (2021) A review on the failure and regulation of solid electrolyte interphase in lithium batteries. J Energy Chem 59:306–319
Escorihuela J, Olvera-Mancilla J, Alexandrova L, del Castillo LF, Compañ V (2020) Recent progress in the development of composite membranes based on polybenzimidazole for high temperature proton exchange membrane (PEM) fuel cell applications. Polymers 12:1861
Estager J, Holbrey JD, Swadźba-Kwaśny M (2014) Halometallate ionic liquids-revisited. Chem Soc Rev 43:847–886
Fan L, Tu Z, Chan SH (2021) Recent development of hydrogen and fuel cell technologies: a review. Energy Rep 7:8421–8446
Fang Y, Xiang W, Zhou X, Lin Y, Fang S (2011) High-performance novel acidic ionic liquid polymer/ionic liquid composite polymer electrolyte for dye-sensitized solar cells. Electrochem Commun 13:60–63
Farobie O, Hartulistiyoso E (2022) Palm oil biodiesel as a renewable energy resource in Indonesia: current status and challenges. Bioenerg Res 15(1):93–111
Fedorov RG, Maletti S, Heubner C, Michaelis A, Ein-Eli Y (2021) Molecular engineering approaches to fabricate artificial solid-electrolyte interphases on anodes for Li-ion batteries: a critical review. Adv Energy Mater 11(26):2101173
Ferreira TA, Flores-Aguilar JF, Santos EM, Rodriguez JA, Ibarra IS (2019) New poly (ionic liquid) based fiber for determination of oxytetracycline in milk samples by application of SPME-CE technique. Molecules 24(3):430
Ganesan S, Muthuraaman B, Mathew V, Madhavan J, Maruthamuthu P, Austin SS (2008) Performance of a new polymer electrolyte incorporated with diphenylamine in nanocrystalline dye-sensitized solar cell. Sol Energy Mater Sol Cells 92:1718–1722
Gao MR, Xu YF, Jiang J, Yu SH (2013) Nanostructured metal chalcogenides: synthesis, modification, and applications in energy conversion and storage devices. Chem Soc Rev 42(7):2986–3017
Garche J, Moseley PT, Karden E (2015) Lead–acid batteries for hybrid electric vehicles and battery electric vehicles. In: Advances in battery technologies for electric vehicles (pp. 75–101). Woodhead Publishing
Greaves TL, Drummond CJ (2008) ChemInform abstract: protic ionic liquids: properties and applications. ChemInform 39:206–237
Hadjipaschalis I, Poullikkas A, Efthimiou V (2009) Overview of current and future energy storage technologies for electric power applications. Renew Sustain Energy Rev 13:1513–1522
Hallett JP, Welton T (2011) Room-temperature ionic liquids: solvents for synthesis and catalysis. Chem Rev 111:3508–3576
Han H, Jiang T, Wu T, Yang D, Han B (2015) VxOy supported on hydrophobic poly (ionic liquid) s as an efficient catalyst for direct hydroxylation of benzene to phenol. ChemCatChem 7(21):3526–3532
Haque M, Li Q, Kuzmenko V, Smith AD, Enoksson P (2017) Ionic liquid electrolyte for supercapacitor with high temperature compatibility. J Phys Conf Ser 922:2–8
Hernandez-Martinez AR, Estevez M, Vargas S, Quintanilla F, Rodriguez R (2011) New dye-sensitized solar cells obtained from extracted bracts of Bougainvillea glabra and spectabilis betalain pigments by different purification processes. Int J Mol Sci 12:5565–5576
Ho MY, Khiew PS, Isa D, Tan TK, Chiu WS, Chia CH (2014) A review of metal oxide composite electrode materials for electrochemical capacitors. NANO 9:1–25
Iimori T, Iwahashi T, Kanal K, Seki K, Sung J, Kim D, Hamaguchi HO, Ouchi Y (2007) Local structure at the air/liquid interface of room-temperature ionic liquids probed by infrared-visible sum frequency generation vibrational spectroscopy: L-alkyl-3-methylimidazolium tetrafluoroborates. J Phys Chem B 111:4860–4866
Jiang W, Li X, Gao G, Wu F, Luo C, Zhang L. (2022) Advances in applications of ionic liquids for phase change CO2 capture. Chem Eng J 136767
Jonathan E, Onimisi M, Eli D, Abdu S, Abdulsalam M (2016) Photovoltaic perfomance of dye sensitized solar cells using natural dyes extracted from bougainvillea flower and mango leaves. J Sci Res Rep 10(6):1–5
Kale SB, Nirmale TC, Khupse ND, Kale BB, Kulkarni MV, Pavitran S, Gosavi SW (2021) Cellulose-derived flame-retardant solid polymer electrolyte for lithium-ion batteries. ACS Sustain Chem Eng 9(4):1559–1567
Kang MG, Ryu KS, Chang SH, Park NG (2004) A new ionic liquid for a redox electrolyte of dye-sensitized solar cells. ETRI J 26(647):652
Karim MR, Islam A, Singh SP, Han L (2011) Quasi solid-state dye-sensitized solar cell incorporating highly conducting polythiophene-coated carbon nanotube composites in ionic liquid. Adv Optoelectron Article ID 357974:1–7
Kato T, Kado T, Tanaka S, Okazaki A, Hayase S (2006a) Quasi-solid dye-sensitized solar cells containing nanoparticles modified with ionic liquid-type molecules. J Electrochem Soc 153:A626
Kato T, Okazaki A, Hayase S (2006b) Latent gel electrolyte precursors for quasi-solid dye sensitized solar cells the comparison of nano-particle cross-linkers with polymer cross-linkers. J Photochem Photobiol A Chem 179:42–48
Kianfar E, Mafi S (2020) Ionic liquids: properties, application, and synthesis. Fine Chem Eng: 22–31
Kim H, Kim H, Ding Z, Lee MH, Lim K, Yoon G, Kang K (2016) Recent progress in electrode materials for sodium-ion batteries. Adv Energy Mater 6(19):1600943
Kitazawa Y, Iwata K, Kido R, Imaizumi S, Tsuzuki S, Shinoda W, ... Watanabe M (2018) Polymer electrolytes containing solvate ionic liquids: a new approach to achieve high ionic conductivity, thermal stability, and a wide potential window. Chem Mater 30(1):252–261
Klass DL (2004) Biomass for renewable energy and fuels. Encycl Energy 1(1):193–212
Koasidis K, Nikas A, Neofytou H, Karamaneas A, Gambhir A, Wachsmuth J, Doukas H (2020) The UK and German low-carbon industry transitions from a sectoral innovation and system failures perspective. Energies 13(19):4994
Kraytsberg A, Ein-Eli Y (2014) Review of advanced materials for proton exchange membrane fuel cells. Energy Fuels 28(12):7303–7330
Kuang D, Comte P, Zakeeruddin SM, Hagberg DP, Karlsson KM, Sun L, Nazeeruddin MK, Grätzel M (2011) Stable dye-sensitized solar cells based on organic chromophores and ionic liquid electrolyte. Sol Energy 85:1189–1194
Kumar MS, Revankar ST (2017) Development scheme and key technology of an electric vehicle: an overview. Renew Sustain Energy Rev 70:1266–1285
Kusama H, Arakawa H (2004) Influence of benzimidazole additives in electrolytic solution on dye-sensitized solar cell performance. J Photochem Photobiol A Chem 162:441–448
Kushwaha R, Srivastava P, Bahadur L (2013) Natural pigments from plants used as sensitizers for TiO 2 based dye-sensitized solar cells. J Energy 2013:1–8
Kwak WJ, Rosy Sharon D, Xia C, Kim H, Johnson LR, ... Aurbach D (2020) Lithium–oxygen batteries and related systems: potential, status, and future. Chem Rev 120(14):6626–6683
Kwon HN, Jang SJ, Kang YC, Roh KC (2019) The effect of ILs as Co-salts in electrolytes for high voltage supercapacitors. Sci Rep 9:1–6
Lee HJ, Kim WS, Park SH, Shin WS, Jin SH, Lee JK, Han SM, Jung KS, Kim MR (2006) Effects of nanocrystalline porous TiO2 films on interface adsorption of phthalocyanines and polymer electrolytes in dye-sensitized solar cells. Macromol Symp 235:230–236
Li M, Lu J, Chen Z, Amine K (2018a) 30 years of lithium-ion batteries. Adv Mater 30(33):1800561
Li M, Zhao C, Wang ZK, Zhang CC, Lee HKH, Pockett A, Barbé J, Tsoi WC, Yang YG, Carnie MJ, Gao XY, Yang WX, Durrant JR, Liao LS, Jain SM (2018b) Interface modification by ionic liquid: a promising candidate for indoor light harvesting and stability improvement of planar perovskite solar cells. Adv Energy Mater 8:1–8
Lin Z, Taberna PL, Simon P (2016) Graphene-based supercapacitors using eutectic ionic liquid mixture electrolyte. Electrochim Acta 206:446–451
Macfarlane DR, Tachikawa N, Forsyth M, Pringle JM, Howlett PC, Elliott GD, Davis JH, Watanabe M, Simon P, Angell CA (2014) Energy applications of ionic liquids. Energy Environ Sci 7:232–250
Mansuroglu A, Arvas MB, Kiraz C, Sayhan B, Akgumus A, Gencten M, Sahin Y (2021) N-doped graphene oxide as additive for fumed silica based gel electrolyte of valve regulated lead acid batteries. J Electrochem Soc 168(6):060512
Marom R, Amalraj SF, Leifer N, Jacob D, Aurbach D (2011) A review of advanced and practical lithium battery materials. J Mater Chem 21(27):9938–9954
Meda US, Lal L, Sushantha M, Garg P (2021) Solid electrolyte interphase (SEI), a boon or a bane for lithium batteries: a review on the recent advances. J Energy Storage 103564
Meyerowitz ESC, EM (1991) Nature Publishing Group, Nature 354:56–58
Miao Q, Zhang S, Xu H, Zhang P, Li H (2013) A novel ionic liquid–metal complex electrolyte for a remarkable increase in the efficiency of dye-sensitized solar cells. Chem Commun 49:6980–6982
Najib S, Erdem E (2019) Current progress achieved in novel materials for supercapacitor electrodes: mini review. Nanoscale Adv 1:2817–2827
Nei De Freitas J, Nogueira AF, De Paoli MA (2009) New insights into dye-sensitized solar cells with polymer electrolytes. J Mater Chem 19:5279–5294
Newell R, Faure-Vincent J, Iliev B, Schubert T, Aradilla D (2018) A new high performance ionic liquid mixture electrolyte for large temperature range supercapacitor applications (−70 °C to 80 °C) operating at 3.5V cell voltage. Electrochim Acta 267:15–19
Nguyen HP, Hoang AT, Nizetic S, Nguyen XP, Le AT, Luong CN, Pham VV (2021) The electric propulsion system as a green solution for management strategy of CO2 emission in ocean shipping: a comprehensive review. Int Transact Electric Energy Syst 31(11):e12580
Ning C, Jing Z, Difei Z, Zhihui Y, Jin G, Peng W (2009) N-Methyl-N-allylpyrrolidinium based ionic liquids for solvent-free dye-sensitized solar cells. J Phys Chem C 113:4215–4221
Pandey S (2006) Analytical applications of room-temperature ionic liquids: a review of recent efforts. Anal Chim Acta 556:38–45
Papageorgiou N, Athanassov Y, Armand M, Bonho⁁te P, Pettersson H, Azam A, Grätzel M (1996) The performance and stability of ambient temperature molten salts for solar cell applications. J Electrochem Soc 143(3099):3108
Placke T, Kloepsch R, Dühnen S, Winter M (2017) Lithium ion, lithium metal, and alternative rechargeable battery technologies: the odyssey for high energy density. J Solid State Electrochem 21(7):1939–1964
Qazi UY (2022) Future of hydrogen as an alternative fuel for next-generation industrial applications; challenges and expected opportunities. Energies 15(13):4741
Qin D, Zhang Y, Huang S, Luo Y, Li D, Meng Q (2011) Ionic liquid/polymer composite electrolytes by in situ photopolymerization and their application in dye-sensitized solar cells. Electrochim Acta 56:8680–8687
Rantwijk F, Van Sheldon RA (2007) Cr050946x-OK.Pdf. Chem Rev 107:2757–2785
Ray A, Saruhan B (2021) Application of ionic liquids for batteries and supercapacitors. Materials 14(11):2942
Sadati Sorkhi SE, Mahmoud Hashemi M (2022) Introduction of a novel dicationic Brönsted acidic ionic liquid based on pyrazine and its application in the synthesis of xanthenediones and 3, 4-dihydropyrimidin-2 (1H)-ones under solvent-free conditions. Razi J Med Sci 29(7): 0–0
Saleem AM, Desmaris V, Enoksson P (2016) Performance enhancement of carbon nanomaterials for supercapacitors. J Nanomater Article ID 1537269: 1–17
Samantara AK, Ratha S (2018) Components of Supercapacitor 1:11–39
Sampaio AM, Fileti EE, Siqueira LJA (2019) Atomistic study of the physical properties of sulfonium-based ionic liquids as electrolyte for supercapacitors. J Mol Liq 296:112065
Santa-Nokki H, Busi S, Kallioinen J, Lahtinen M, Korppi-Tommola J (2007) Quaternary ammonium polyiodides as ionic liquid/soft solid electrolytes in dye-sensitized solar cells. J Photochem Photobiol A Chem 186:29–33
Santos F, Ivanou D, Mendes A (2022) The Renaissance of monolithic dye-sensitized solar cells. Mater Today Commun 104030
Seddon KR (2003) A taste of the future. Nat Mater 2:363–365
Selcuk B (2014) Structure and environmental impact of global energy consumption. Renew Sustain Energy Rev 38:890–902
Seng LK, Masdar MS, Shyuan LK (2021) Ionic liquid in phosphoric acid-doped polybenzimidazole (PA-PBI) as electrolyte membranes for PEM fuel cells: a review. Membranes 11(10):728
Seo JY, Matsui T, Luo J, Correa-Baena JP, Giordano F, Saliba M, Schenk K, Ummadisingu A, Domanski K, Hadadian M, Hagfeldt A, Zakeeruddin SM, Steiner U, Grätzel M, Abate A (2016a) Ionic liquid control crystal growth to enhance planar perovskite solar cells efficiency. Adv Energy Mater 6:1–6
Seo JY, Matsui T, Luo J, Correa-Baena JP, Giordano F, Saliba M, Schenk K, Ummadisingu A, Domanski K, Hadadian M, Hagfeldt A, Zakeeruddin SM, Steiner U, Grätzel M, Abate A (2016b) Ionic liquid control crystal growth to enhance planar perovskite solar cells efficiency. Adv Energy Mater 6:1–6
Shahzad S, Shah A, Kowsari E, Iftikhar FJ, Nawab A, Piro B, Akhter MS, Rana UA, Shibl HM, Hafez HS, Rifai RI (2013) Abdel Mottaleb, M. S. A. Environmental friendly, low cost quasi solid state dye sensitized solar cell: polymer electrolyte introduction. J Inorg Organomet Polym Mater 23:944–949
Shahzad S, Shah A, Kowsari E, Iftikhar FJ, Nawab A, Piro B, Akhter MS, Rana UA, Zou Y (2019) Ionic liquids as environmentally benign electrolytes for high-performance supercapacitors. Glob Challenges 3:1800023
Simon P, Gogotsi Y, Dunn B (2014) Where do batteries end and supercapacitors begin? Science 343:1210–1211
Singh K, Ohlan A, Saini P, Dhawan SK (2008) Composite – super paramagnetic behavior and variable range hopping 1D conduction mechanism – synthesis and characterization. Polym Adv Technol 2007:229–236
Smitha B, Sridhar S, Khan AA (2005) Solid polymer electrolyte membranes for fuel cell applications—a review. J Membr Sci 259(1–2):10–26
Tang X, Lv S, Jiang K, Zhou G, Liu X (2022) Recent development of ionic liquid-based electrolytes in lithium-ion batteries. J Power Sources 542:231792
Tokuda H, Hayamizu K, Ishii K, Susan MABH (2004) Watanabe, M. Physicochemical properties and structures of room temperature ionic liquids. 1. Variation of anionic species. J Phys Chem B 108:16593–16600
Tõnurist K, Thomberg T, Jänes A, Kink I, Lust E (2012) Specific performance of electrical double layer capacitors based on different separator materials in room temperature ionic liquid. Electrochem Commun 22:77–80
Vangari M, Pryor T, Jiang L (2013) Supercapacitors: review of materials and fabrication methods. J Energy Eng 139:72–79
Wang P, Zakeeruddin SM, Exnar I, Grtzel M (2002) High efficiency dye-sensitized nanocrystalline solar cells based on ionic liquid polymer gel electrolyte electronic supplementary information (ESI) available: fabrication procedure for the DSSCs. Chem Commun 5:2972–2973
Wang P, Zakeeruddin SM, Comte P, Exnar I, Grätzel M (2003a) Gelation of ionic liquid-based electrolytes with silica nanoparticles for quasi-solid-state dye-sensitized solar cells. J Am Chem Soc 125:1166–1167
Wang P, Zakeeruddin SM, Moser JE, Grätzel MA (2003b) New ionic liquid electrolyte enhances the conversion efficiency of dye-sensitized solar cells. J Phys Chem B 107:13280–13285
Wang N, Lin H, Li J, Li X (2006) Improved quasi-solid dye-sensitized solar cells by composite ionic liquid electrolyte including layered α -zirconium phosphate. Appl Phys Lett 89:19
Wang M, Xiao X, Zhou X, Li X, Lin Y (2007) Investigation of PEO-imidazole ionic liquid oligomer electrolytes for dye-sensitized solar cells. Sol Energy Mater Sol Cells 91:785–790
Wang YT, Yang XX, Xu J, Wang HL, Wang ZB, Zhang L, Liang JL (2019) Biodiesel production from esterification of oleic acid by a sulfonated magnetic solid acid catalyst. Renew Energy 139: 688–695
Wasserscheid P (2006) Volatile times for ionic liquids. Nature 439:797
Wilkes JS (2002) A short history of ionic liquids - from molten salts to neoteric solvents. Green Chem 4:73–80
Wilkes JS, Zaworotko M (1992) Air and water stable 1-ethyl-3-methylimidazolium based ionic liquids. J Chem Soc Chem Commun 13:965–967
Wilkes JS, Levisky JA, Wilson RA, Hussey CL (1982) Dialkylimidazolium chloroaluminate melts: a new class of room-temperature ionic liquids for electrochemistry, spectroscopy and synthesis. Inorg Chem 2:1263–1264
Wu Q, Zhou W, Liu Q, Zhou P, Chen T, LuY QQ, Yang S (2016) Solution-processable ionic liquid as an independent or modifying electron transport layer for high-efficiency perovskite solar cells. ACS Appl Mater Interfaces 8:34464–34473
Wu J, Yuan L, Zhang W, Li Z, Xie X, Huang Y (2021a) Reducing the thickness of solid-state electrolyte membranes for high-energy lithium batteries. Energy Environ Sci 14(1):12–36
Wu W, Bai Y, Wang X, Wu C (2021b) Sulfone-based high-voltage electrolytes for high energy density rechargeable lithium batteries: progress and perspective. Chin Chem Lett 32(4):1309–1315
Yang D, Yang R, Ren X, Zhu X, Yang Z, Li C, Liu SF (2016) Hysteresis-suppressed high-efficiency flexible perovskite solar cells using solid-state ionic-liquids for effective electron transport. Adv Mater 28:5206–5213
Yang Q, Zhang Z, Sun XG, Hu YS, Xing H, Dai S (2018) Ionic liquids and derived materials for lithium and sodium batteries. Chem Soc Rev 47(6):2020–2064
Yao Y, Zhang E, Xia X, Yu J, Wu K, Zhang Y, Wang H (2015) Morphology and properties of cellulose/silk fibroin blend fiber prepared with 1-butyl-3-methylimidazolium chloride as solvent. Cellulose 22(1):625–635
Yin B, Wu Y, Liu C, Wang P, Wang L, Sun G (2021) An effective strategy for the preparation of a wide-temperature-range proton exchange membrane based on polybenzimidazoles and polyacrylamide hydrogels. J Mater Chem A9(6):3605–3615
Yue C, Sun P, Li F (2020) Sulfonium ionic liquids BT - encyclopedia of ionic liquids. Zhang S (Ed.); Springer Singapore: Singapore, pp 1–14
Zhang Y, Fei Z, Gao P, Lee Y, Tirani FF, Scopelliti R, Feng Y, Dyson PJ, Nazeeruddin MK (2017) A strategy to produce high efficiency, high stability perovskite solar cells using functionalized ionic liquid-dopants. Adv Mater 29:1–8
Zhang H, Zhang J, Ma J, Xu G, Dong T, Cui G (2019) Polymer electrolytes for high energy density ternary cathode material-based lithium batteries. Electrochem Energ Rev 2(1):128–148
Zhang J, Zhang X, Yang M, Singh S, & Cheng G (2021) Transforming lignocellulosic biomass into biofuels enabled by ionic liquid pretreatment. Bioresource Technology 322:124522
Zhong C, Deng Y, Hu W, Qiao J, Zhang L, Zhang J (2015) A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem Soc Rev 44:7484–7539
Acknowledgements
We express our sincere gratitude to the Head and Dean, Department of Chemistry, Central University of Allahabad, Prayagraj, for providing laboratory facility.
Author information
Authors and Affiliations
Contributions
Manoj K. Banjare, Rahul Kanaoujiya, Shruti Trivedi, and Kamalakanta Behera: conceptualization and supervision and review and editing. Gaurav Choudhary, Jyoti Dhariwal, and Moumita Saha: review and editing, original draft preparation, and visualization. All the authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: George Z. Kyzas
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Choudhary, G., Dhariwal, J., Saha, M. et al. Ionic liquids: environmentally sustainable materials for energy conversion and storage applications. Environ Sci Pollut Res 31, 10296–10316 (2024). https://doi.org/10.1007/s11356-023-25468-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-25468-w