Abstract
Myopia is one of the most common forms of refractive eye disease and considered as a worldwide pandemic experienced by half of the global population by 2050. During the past several decades, myopia has become a leading cause of visual impairment, whereas several factors are believed to be associated with its occurrence and development. In terms of environmental factors, air pollution has gained more attention in recent years, as exposure to ambient air pollution seems to increase peripheral hyperopia defocus, affect the dopamine pathways, and cause retinal ischemia. In this review, we highlight epidemiological evidence and potential biological mechanisms that may link exposure to air pollutants to myopia. A thorough understanding of these mechanisms is a key for establishing and implementing targeting strategies. Regulatory efforts to control air pollution through effective policies and limit individual exposure to preventable risks are required in reducing this global public health burden.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
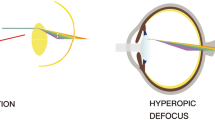
Myopia (short-sightedness or near-sightedness) is defined as a spherical equivalent (SE) ≤ − 0.5diopters (D)(Baird et al. 2020). The World Health Organization (WHO) recognizes that individuals with myopia are at a substantially increased risk of potentially blinding myopic pathologies that are not prevented by optical correction (Morgan et al. 2012). The pathologic complications include myopic macular degeneration, choroidal neovascularization, cataract, and glaucoma (Pan et al. 2012). For more than one third of adults, myopia can progress during the third decade of life (Lee et al. 2022). The continuous progression of myopia may result in significant reductions in work, educational productivity, and overall quality of life. However, approximately 2 billion individuals (28.3% of the global population) are diagnosed with myopia worldwide, and its prevalence is estimated to increase to 4.76 billion individuals (49.8% of the global population) by 2050 (Baird et al. 2020; Morgan et al. 2018). The cumulative incidence of myopia among Chinese school-aged children and adolescents has increased consistently, reaching 91.3% (minimum to maximum: 83.7–96.7%) upon graduation from high school (Chen et al. 2021). The causes of this pandemic remain unclear. The commonly agreed underlying mechanisms are peripheral hyperopia defocus, dopamine (DA) pathway, and retinal ischemia (Fig. 1). Such mechanisms are affected by air pollutants and may potentially link myopia to air pollution.
The underlying pathways of myopia pathogenesis. The clear “123” indicates the vision of emmetropia, while the blurred one represents myopia. When the peripheral hyperopia defocus occurs, the incoming light rays are focused behind the retina surface and axial growth is lengthened owing to the tissue remodeling of the sclera. The axial elongation can also be promoted by the hypoxia marker upregulated by retinal ischemia, while inhibited by the release of retinal dopamine. DA dopamine
Air pollution is a complex mixture of gaseous components and particulate matter (PM) suspended in air (Miyazaki et al. 2020). Gaseous pollutants include carbon monoxide (CO), nitrogen oxides (NOx), and ozone (O3) (Ruan et al. 2019). Ambient PMs are emitted by the combustion of fossil fuels or natural sources, such as volcanic eruptions and wildfires (Miyazaki et al. 2020). PMs are categorized by their diameter: particulate matter 10 (PM10), particulate matter 2.5 (PM2.5), and ultrafine PMs (Miyazaki et al. 2020; Gao et al. 2019). A report from the Organization for Economic Co-operation and Development indicated that outdoor air pollution could cost $2.6 trillion a year, by 2060 (Bai et al. 2018). According to the WHO, approximately seven million individuals die from air pollution annually (Areal et al. 2022). There are regions of the world, notably in Asia, that bear the greatest disease burden from air pollution (The 2017). Exposure to air pollution has been associated with asthma, cognitive functioning, neurodegenerative diseases, dry eye disease, blepharitis, conjunctivitis, and cataracts (Orru et al. 2017; Schraufnagel et al. 2019). In fact, many epidemiological evidences have proved greater air pollutant concentrations to be strongly associated with an increased likelihood of myopia (Yang et al. 2021; Ruan et al. 2019; Wei et al. 2019; Dadvand et al. 2017).
In this review, we summarize the currently available data on the association between air pollution and myopia for the lack of a comprehensive review on this topic so far. We aim to explore the underlying pathophysiological mechanisms hoping to inspire future research and provide more insights into the clean-air regulation efforts for governments.
Epidemiological evidence of ambient air pollutant exposure on myopia
Several air pollutants are positively correlated with the incidence of myopia (Ruan et al. 2019; Wei et al. 2019; Dadvand et al. 2017; Yang et al. 2021), as shown in Table 1. PM2.5, particles ≤ 2.5 µm in aerodynamic diameter, are primarily produced by motor vehicles, power plants, and other combustion sources (Adar et al. 2010). The small size of PM2.5 makes them particularly deleterious to health, causing respiratory and cardiovascular morbidity. Wei et al. (2019) conducted a retrospective cohort study including 97,306 children aged 6–12 years and found that those who were exposed to higher PM2.5 concentrations had a higher cumulative incidence of myopia. A cohort study of 61,995 children in 7 Chinese provinces/municipalities reported the associations of PMs between prevalent visual impairment and visual acuity levels (Yang et al. 2021). Similar observations made by Dadvand et al. (2017) suggested that there was an increase in the likelihood of myopia (as surrogated by spectacle use) associated with exposure to residential PM2.5 absorbance. To confirm the relationship between PM2.5 and myopia, Wei et al. (2019) treated 3-week-old hamsters with 100 μg/mL PM2.5 twice a day. After 3 weeks, the hamsters developed myopia (change in refractive error = − 1.513 ± 2.092 D), whereas the hamsters in the control and PM2.5 + resveratrol groups did not (change in refractive error = 0.913 ± 1.772 D and 0.65 ± 1.36 D, respectively).
NOx is a collective expression of nitric oxide (NO) and nitrogen dioxide (NO2), which transform into each other in the atmosphere. They are primarily emitted from power stations, motor vehicles, and other industrial combustion processes (Gao et al. 2022). Yang et al. reported that long-term exposure to NO2 was associated with increased odds of visual impairment (Yang et al. 2021). The cross-sectional analyses conducted by Dadvand et al. (2017) observed that an increase in NO2 levels at home and school was associated with an increase in spectacle use and a higher risk of myopia. Similar conclusions were drawn for NOx concentrations (Wei et al. 2019).
Ground-level O3 is a typical secondary pollutant produced by photochemical reactions and is a potent greenhouse gas with both direct and indirect effects on human health (Luo et al. 2021; Xu et al. 2022; Montes et al. 2022). Ruan et al. (2019) discovered a synergistic interaction of two air pollutants on myopia, and the joint effect of high PM2.5 and high O3 on myopia (95% confidence interval (CI): 1.23, 1.73) was greater than the sum of their individual effects with a synergistic index of 1.81 (95% CI: 0.92, 4.94).
Although current studies are limited and include different air pollutants, they have all revealed a strong correlation between air pollution and myopia.
Potential biological mechanisms of air pollutants on myopia
Eyes are exposed to ambient air pollution, making them prime vulnerable targets for the adverse effects of such exposure. O3 is linked to the overexpression of conjunctival interleukin (IL)-6 and tumor necrosis factor (TNF)-α (Jung et al. 2018), which are two typical inflammatory cytokines that initiate ocular inflammation. PM and NOx can stimulate the formation of reactive oxygen species (ROS) and free radicals, which lead to oxidative stress (Lasagni Vitar et al. 2019; Wei et al. 2019). Prolonged elevated levels of ROS can result in redox disruption of metabolic, signaling, and transcription processes, which cause oxidative damage to macromolecules in both the cornea and retina (Lasagni Vitar et al. 2019; Liu et al. 2020; Ying et al. 2021).
In this case, the potential pathophysiology of air pollutants on myopia is classified into direct and indirect ways. The direct pathway refers to the direct distribution and concentration of pollutants on the eye and airway, leading to enhanced inflammation which is closely related to peripheral hyperopic scatter and retinal ischemia. The indirect one refers to the decreased release of DA in the eyes caused by less outdoor light and more air pollution.
Indirect pathway: the DA pathway
DA is a neurotransmitter used by a class of amacrine cells that plays an important role in the retina, mediating eye functions such as visual signaling, ocular development, and refractive adjustment (Zhang and Deng 2020; Norton 2016; Zhou et al. 2017). Previous experiments in multiple species have suggested that DA acts as a “stop” signal in progression of myopia. Animal models, including chicks, mice, and primates, have demonstrated that dopaminergic compound administration can retard ocular growth by slowing vitreal chamber elongation and significantly inhibit the development of myopia in a similar dose-dependent manner (Thomson et al. 2020b, 2020a). In mice, this regulation may involve D2 receptors, a DA receptor subtype located in the retina (Huang et al. 2022; Thomson et al. 2020b). The release of retinal DA can participate in the retina-to-sclera signaling cascade, which induces scleral remodeling in response to sunlight stimuli (Grzybowski et al. 2020).
As the main component of sunlight, cumulative ultraviolet (UV) exposure can influence the presence of myopia (Kearney et al. 2019; Williams et al. 2017) through the DA pathways, particularly in adolescence and young adulthood (Williams et al. 2017). In rabbits, the abnormal elongation of the myopic eye was effectively controlled 1 month after ultraviolet A (UVA; a band of UV whose wavelengths range from 315 to 400 nm (Bajgar et al. 2021)) irradiation and almost halted 3 months after treatment (Rong et al. 2017). Similarly, posterior scleral cross-linking induced by riboflavin-UVA can slow the progression of myopia (Han et al. 2021; Lai et al. 2021; Dotan et al. 2016; Li et al. 2017). Serum metabolomic and lipidomic studies carried out by Du et al. (2020) revealed five pathways showing regulatory relationships with D2Rs in myopia: steroid biosynthesis, arginine and proline metabolism, linoleic acid metabolism, alpha-linolenic acid metabolism, and sphingolipid metabolism. Kato et al. (2019) suggested that UVA absorption had a direct effect on fibroblasts of the sclera and cornea. Consequently, solar UV radiation can be related to a change in layer thickness and/or rearrangement inside axons in the nerve fiber layer, as well as the connectivity between several different cell types in the retina to slow down myopic eye growth (Landis et al. 2021; Wirz-Justice et al. 2021; Lingham et al. 2020; Swiatczak et al. 2019).
The reduction in UV exposure that reaches the Earth’s surface occurs during the synthesis and emission of air pollutants (Borysov et al. 2020; Kalluri et al. 2021; Manisalidis et al. 2020, United Nations Environment Programme 2016). It is acknowledged that PM2.5 in the atmosphere can directly reflect solar radiation back into space because the physical properties of particles are strongly curved interfaces (Riva et al. 2021). Black carbon (BC) emitted by wildfires across the globe can persist in the atmosphere for days to weeks owing to its highly adhesive surface (Borysov et al. 2020). Such persistent increases in carbonaceous aerosols would significantly reduce UV radiation by chemical processes, such as oxidation and light-catalyzed reaction (Manisalidis et al. 2020; Guo et al. 2021; United Nations Environment Programme 2016). Owing to the impact of air pollution on UV exposure, it is reasonable to assume that air pollution can lead to myopia through the DA pathways affected by reduced UV exposure.
Direct pathways
Peripheral hyperopia defocus
Peripheral hyperopia defocus indicates that peripheral images are focused behind the retinal surface, whereas the foveal image falls exactly on the retina (Rotolo et al. 2017). Animal experiments have proposed a mechanism in which the retina can “read” the direction of focus of incoming light rays on the retina and affect the choroid to actively change its thickness to move the retina towards the image plane via changes in retinal homeostasis mediated by neurotransmitters (Kubota et al. 2021). If the focal plane is behind the retina in areas of the visual field, axial growth is promoted owing to thinning of the subfoveal choroid (Schaeffel 2017; Kubota et al. 2021). Accumulating evidence has demonstrated that alterations of the focal plane can be related to ocular surface diseases, and the biophysical property changes of corneal cells involved in these diseases may reflect myopia progression, as the cornea contributes more than 60% of the focusing power (Xin et al. 2021).
Previous investigations have revealed a significant association between continuously increasing air pollution and ocular surface disorders, such as uveitis (Bai et al. 2021), keratitis (Sendra et al. 2021), and conjunctivitis (Antonini et al. 2021; Nucci et al. 2017). NO is converted to NO2, followed by the generation of O3 (Miyazaki et al. 2019). NO2 and O3 can directly damage the ocular surface by oxidation (Hong et al. 2016), acidification of tears, allergic sensitization, and chemical modification of aeroallergens (Lu et al. 2021). Allergic conjunctivitis (AC) is a common ocular surface disease that causes dry eye, itching, and burning sensations, and may lead to sight-threatening conditions (Lu et al. 2021). Reports from highly prevalent regions of AC suggest that air pollutants may be associated with increased sensitization (Miyazaki et al. 2020) and can induce or aggravate AC symptoms. Lu et al. (2021) found that the incidence of AC was positively correlated with PM2.5, PM10, CO, NO2, and O3. The number of outpatient visits with AC increased as the concentrations of NO2 (Hong et al. 2016; Wei et al. 2019; Miyazaki et al. 2020), O3 (Hong et al. 2016; Miyazaki et al. 2020), PM2.5 (Mu et al. 2020; Miyazaki et al. 2020), and PM10 (Miyazaki et al. 2020) changed. A correlation between allergic inflammation and subsequent myopia risk has also been established (Lin et al. 2016).
In patients with AC, immune-mediated destruction of the conjunctival epithelium is triggered when the ocular surface encounters environmental antigens. Subsequently, activated T cells and mast cells would specifically target sensitized ocular surface tissues, reduce mucin-secreting cells and break down integrity (Jung et al. 2018; Wei et al. 2018). Additionally, PM and NOx have been suggested to alter the barrier integrity of the cornea due to the induced increase in ROS (Wei et al. 2019; Lasagni Vitar et al. 2019). Overloading of the antioxidative defense system in the conjunctiva leads to the production of inflammatory cytokines (Wolkoff 2017). Wei et al. (2018) verified that TNF-α and IL-6 reduced the levels of claudin-1 and zonula occludens-1 tight junction proteins in the barrier of corneal epithelial cells. Consequently, elevated inflammatory mediator release and corneal injury caused by AC have been proposed as possible mechanisms for increased corneal curvature. Wei et al. (2018) performed a cohort study and confirmed that children with AC had a 2.35-fold higher incidence of myopia than those without AC. They also observed that the rats with AC developed myopia. Additionally, the axial lengths of the eyes with AC were significantly longer than those of the control eyes (Wei et al. 2018). A case–control study conducted by Wang et al. (2021a) reported that patients with AC had an increased risk of keratoconus, which is mainly characterized by progressive corneal thinning and cone-shaped corneal protrusion (Wang et al. 2021a, 2021b; Ahmed et al. 2021). This implies a steeper central cornea and a flatter periphery. The relatively negative spherical aberration in the periphery causes peripheral hyperopia defocus and stimulates eyeball growth (Atchison and Rosén 2016), which, in turn, promotes the development of myopia.
Retinal ischemia
Vascular densities are reported to be significantly negatively correlated with axial length, including the capillaries in the superficial and deep macula, peripapillary area, and choroid (Wang et al. 2021c; Zheng et al. 2015; Liu et al. 2021). Quantitatively measured alterations in retinal vascular diameter may imply a retina abnormality (Chen et al. 2017). The decrease in microvascular density could conceivably lead to reduced metabolic demands in the local retinal region because it serves as a direct source of oxygen and nutrients for retinal pigment epithelium cells and retinal nerve fiber layer (RNFL). The hypoxic microenvironment has been shown to upregulate the expression of tissue hypoxia marker (Liu et al. 2021) and reduce the density of retinal pigment epithelium cells in the retro-equatorial region, causing a stretch of axial elongation (Wu et al. 2019). Grudzińska et al. (2022, Zheng et al. 2015) found a positive correlation between peak systolic velocity and end-diastolic velocity in the central retinal artery and mean thickness of the RNFL, ganglion cell, and inner plexus layer, and size of the rim area (the area located between the edge of the disc and the physiological cup containing the neural elements). Consequently, it can induce parapapillary RNFL thinning and impair retinal neuroactivity, which regulates the axial growth of eyes early in life. Additionally, special attention has been paid to the less tortuous retinal vessels, which can also cause hypoperfusion of the retina (Zheng et al. 2015).
Clinical investigations (Table 2) showed an inverse association between air pollution concentrations (measured as PM2.5, PM10, BC, O3, and NO2 concentrations) and central retinal arteriolar diameter, one of the first branches of the ophthalmic artery (Zhang et al. 2018; Baldoncini et al. 2019; La Spina et al. 2016). As for PM2.5, 4607 participants were examined in the analysis conducted by Adar et al. (2010), who found that central retinal artery diameter was negatively associated with increased long- and short-term levels of PM2.5. Provost et al. (2017) confirmed that each 10-µg/m3 increase in same-day exposure to PM2.5 was associated with 0.62 μm (95% CI: − 1.12, − 0.12) narrower retinal arterioles in school-aged children in Belgium. PM10 is a 2.5–10-μm-sized aerosol that contains coarse suspended materials, including pollen, fungal spores, and dust, which can serve as allergens or adjuvants (Miyazaki et al. 2020). Louwies et al. (2016, Louwies et al. 2013) suggested that PM10 exposure was associated with retinal arteriolar narrowing and venular widening. BC, a by-product of fuel combustion and one of the most toxic components of PM (Witters et al. 2021; Rabito et al. 2020), is associated with systemic inflammation and oxidative stress (Louwies et al. 2015). Louwies et al. (2013) indicated that there was a decrease in retinal artery diameter for each 1-µg/m3 increase in BC. They also observed a positive association between retinal venules and BC exposure (Louwies et al. 2015). Additionally, Korsiak et al. (2021) indicated that Ox (the combined oxidant capacity of O3 and NO2 using a redox-weighted average) was inversely associated with retinal arteriolar diameter, and the strongest association was observed for a 7-day mean exposure.
The biological mechanisms underlying the impact of air pollution on the retinal microvasculature are thought to be related to inflammation and oxidative stress pathways. We propose two possible explanations for this finding.
One mechanism is that systemic microvascular endothelium-dependent dilation affected by pulmonary air pollutant exposure, particularly PM, can be related to changes in retinal blood vessels, as they share great similarities in development and anatomy with the microvasculature of the heart, lungs, and brain (Louwies et al. 2013). There is rapidly developing evidence supporting the role of exposure to air pollution in pulmonary inflammation and subsequent low-grade, systemic inflammation (Marchini et al. 2020; Gao et al. 2020; Li et al. 2019). Louwies et al. (2016) suggested a possible role for PM10 in downregulating the expression of microRNAs extracted from venous blood. Differential regulation of microRNAs involved in oxidative stress and inflammatory processes may have contributed to arteriolar narrowing and venular widening. Animals exposed to different types of particles exhibit thickening of alveolar walls, neutrophil recruitment, and macrophage activation, thus increasing systemic oxidative stress and inflammation response, as well as stimulating the generation of ROS and pro-inflammatory cytokines, which finally enter circulation (de Souza Xavier Costa et al. 2020). The substances involved in these systemic reactions may take some time to affect the reactivity of retinal blood vessels, even several hours after exposure, according to Louwies et al. (2013). For instance, ROS that flows to the retina in the bloodstream leads to endothelial nitric oxide synthase uncoupling and reduces the bioavailability of the vasodilator NO, which contributes to endothelial dysfunction and vasoconstriction (Korsiak et al. 2021; Louwies et al. 2013). These alterations in retinal microcirculation, including arteriolar damage and endothelial dysfunction, create a situation of diminished blood flow and impair retinal neuroactivity consequently (Dadvand et al. 2017; De Boever et al. 2014). In turn, it is a source of chronic oxidative stress and inflammation (Chan et al. 2020).
Another hypothesis is that air pollutants can directly affect ocular development and retinal activity owing to their water solubility, concentration, and ability to oxidize tissues. Ultrafine PMs that can pass through the capillary membrane are readily picked up by cells and induce cellular damage (Schikowski 2022). CO emitted from diesel engine exhaust fumes and traffic congestion (Supharakonsakun et al. 2020) is highly soluble, non-irritating, and readily passes through the bloodstream. The toxicity of CO results from its successful competition with oxygen in binding with hemoglobin, decreasing blood oxygen delivery, and resulting in acute tissue hypoxia (Schikowski 2022; Bertrand et al. 2020), whereas NO also attaches to the hemoglobin and other iron-containing proteins because of its binding affinity (Schikowski 2022). Moreover, as gaso-transmitters in vivo, CO and NO transgress cells and tissues rapidly and react with reactive chemical species causing abnormal biochemical reactions (Mahan 2020). However, whether air pollutants directly participate in hypoxic vasoconstriction requires further investigation.
Additionally, air pollution exposure may trigger an autonomic imbalance that favors a sympathetic response to the smooth muscles surrounding the blood vessels and affects retinal vascular densities (Korsiak et al. 2021; Koch et al. 2020). However, it is still controversial because it does not consider that retinal blood vessels lack functional sympathetic innervations (Louwies et al. 2013).
Other evidence linking air pollution to myopia
Other evidence, in addition to the current studies, suggests that the inflammatory response caused by long-term exposure to air pollution induces local biochemical reactions, resulting in direct tissue remodeling and progression of myopia. For example, matrix metalloproteinase-2 (MMP-2) is a scleral extracellular matrix degradation enzyme, and its activation induces collagen fiber I degradation, followed by loss of scleral connective tissue, together with scleral thinning and weakening, which leads to axial elongation and myopia (Ikeda et al. 2020; Lin et al. 2016). Inflammatory cytokines IL-6 and TNF-α in the retina may serve as triggers to initiate MMP-2 activity in the retina, followed by sclera (Yuan et al. 2019), causing progressive scleral remodeling and myopia. Moreover, resveratrol, a naturally occurring antioxidant, has been shown to ameliorate myopia development by blocking the relevant signaling pathways of inflammatory effects above (Hsu et al. 2021; Jiang et al. 2019; Wei et al. 2019).
Conclusion
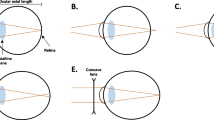
Exposure to ambient air pollution has a significant impact on the development of myopia. Although peripheral hyperopia defocus, the DA pathway, and retinal ischemia are all suggested to play a role (Fig. 2), the detailed mechanisms by which air pollutants interfere with myopia remain unclear and require further investigation. As most of the current epidemiological evidence is cross-sectional in nature, longitudinal studies that comprise a larger scale and quantified measurement of air pollution exposure are required in the future to elucidate the exact impact and provide proof of causality.
Potential mechanisms linking air pollution to myopia. Ambient air pollution may aggravate allergic conjunctivitis symptoms and cause corneal injury, which lead to peripheral hyperopia defocus and stimulate eyeball growth. The synthesis and emission of air pollutants lead to the reduction in ultraviolet exposure and retinal dopamine release. The pulmonary inflammatory factors and reactive oxygen species induced by air pollution can enter the blood circulation, resulting in systemic inflammation and oxidative stress, thus causing retinal ischemia and myopia. Furthermore, several air pollutants may directly induce hypoperfusion of the retina through ocular surface. PM2.5 particulate matter 2.5, CO carbon monoxide, NOx nitrogen oxides, NO nitric oxide, O3 ozone, IL-6 interleukin-6, TNF-α tumor necrosis factor-α, ROS reactive oxygen species, UV ultraviolet, DA dopamine
As aforementioned, air quality is far worse in Asia, and the severity of air pollution may have a substantial role in the etiology of myopia in these countries. Therefore, efforts to address air pollution are required to prevent the incidence and progression of myopia. For policymakers, methods such as establishing and strictly enforcing air quality standards and adopting policies against heavily polluting industries are advised. For the public, it is critical to raise awareness and limit individual exposure to preventable risks such as taking public transportation more.
Availability of data and materials
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
Adar SD, Klein R, Klein BE et al (2010) Air pollution and the microvasculature: a cross-sectional assessment of in vivo retinal images in the population-based multi-ethnic study of atherosclerosis (MESA). PLoS Med [j] 7:e1000372. https://doi.org/10.1371/journal.pmed.1000372
Ahmed AS, El-Agha MH, Khaled MO et al (2021) The prevalence of keratoconus in children with allergic eye disease in an Egyptian population. Eur J Ophthalmol [j] 31:1571–1576. https://doi.org/10.1177/1120672120942691
Antonini M, Gaudenzi D, Spelta S et al (2021) Ocular surface failure in urban syndrome. J Clin Med [j] 10. https://doi.org/10.3390/jcm10143048
Areal AT, Zhao Q, Wigmann C et al (2022) The effect of air pollution when modified by temperature on respiratory health outcomes: a systematic review and meta-analysis. Sci Total Environ [j] 811:152336. https://doi.org/10.1016/j.scitotenv.2021.152336
Atchison DA, Rosén R (2016) The possible role of peripheral refraction in development of myopia. Optom vis Sci [j] 93:1042–1044. https://doi.org/10.1097/opx.0000000000000979
Bai L, Wang J, Ma X et al (2018) Air pollution forecasts: an overview. Int J Environ Res Public Health [j] 15. https://doi.org/10.3390/ijerph15040780
Bai YC, Wang CY, Lin CL et al (2021) Association between air pollution and the risk of uveitis: a nationwide, population-based cohort study. Front Immunol [j] 12:613893. https://doi.org/10.3389/fimmu.2021.613893
Baird PN, Saw SM, Lanca C et al (2020) Myopia. Nat Rev Dis Primers [j] 6:99. https://doi.org/10.1038/s41572-020-00231-4
Bajgar R, Moukova A, Chalupnikova N et al (2021) Differences in the effects of broad-band UVA and narrow-Band UVB on epidermal keratinocytes. Int J Environ Res Public Health [j] 18. https://doi.org/10.3390/ijerph182312480
Baldoncini M, Campero A, Moran G et al (2019) Microsurgical anatomy of the central retinal artery. World Neurosurg [j] 130:e172–e187. https://doi.org/10.1016/j.wneu.2019.06.026
Bertrand L, Dawkins L, Jayaratne R et al (2020) How to choose healthier urban biking routes: CO as a proxy of traffic pollution. Heliyon [j] 6:e04195. https://doi.org/10.1016/j.heliyon.2020.e04195
De Boever P, Louwies T, Provost E, et al. (2014) Fundus photography as a convenient tool to study microvascular responses to cardiovascular disease risk factors in epidemiological studies. J Vis Exp [J] e51904. doi:https://doi.org/10.3791/51904
Borysov A, Tarasenko A, Krisanova N et al (2020) Plastic smoke aerosol: nano-sized particle distribution, absorption/fluorescent properties, dysregulation of oxidative processes and synaptic transmission in rat brain nerve terminals. Environ Pollut [j] 263:114502. https://doi.org/10.1016/j.envpol.2020.114502
Chan TC, Wilkinson Berka JL, Deliyanti D et al (2020) The role of reactive oxygen species in the pathogenesis and treatment of retinal diseases. Exp Eye Res [j] 201:108255. https://doi.org/10.1016/j.exer.2020.108255
Chen P, Cai X, Xu L et al (2017) Assessing oxygen saturation in retinal vessels in high myopia patients pre- and post-implantable collamer lens implantation surgery. Acta Ophthalmol [j] 95:576–582. https://doi.org/10.1111/aos.13368
Chen J, He XG, Wang JJ et al (2021) Forcasting the prevalence of myopia among students aged 6–18 years in China from 2021 to 2030. Zhonghua Yan Ke Za Zhi [j] 57:261–267. https://doi.org/10.3760/cma.j.cn112142-20201228-000851
Dadvand P, Nieuwenhuijsen MJ, Basagaña X et al (2017) Traffic-related air pollution and spectacles use in schoolchildren. PLoS One [j] 12:e0167046. https://doi.org/10.1371/journal.pone.0167046
De Souza Xavier Costa N, Ribeiro Júnior G, Dos Santos Alemany A A et al (2020) Air pollution impairs recovery and tissue remodeling in a murine model of acute lung injury. Sci Rep [J] 10:15314. https://doi.org/10.1038/s41598-020-72130-3
Dotan A, Kremer I, Gal-Or O, et al. (2016) Scleral cross-linking using riboflavin and ultraviolet-A radiation for prevention of axial myopia in a rabbit model. J Vis Exp [J] e53201. doi:https://doi.org/10.3791/53201
Du B, Jin N, Zhu X et al (2020) A prospective study of serum metabolomic and lipidomic changes in myopic children and adolescents. Exp Eye Res [j] 199:108182. https://doi.org/10.1016/j.exer.2020.108182
Gao ZF, Long HM, Dai B et al (2019) Investigation of reducing particulate matter (PM) and heavy metals pollutions by adding a novel additive from metallurgical dust (MD) during coal combustion. J Hazard Mater [j] 373:335–346. https://doi.org/10.1016/j.jhazmat.2019.03.057
Gao N, Xu W, Ji J et al (2020) Lung function and systemic inflammation associated with short-term air pollution exposure in chronic obstructive pulmonary disease patients in Beijing, China. Environ Health [j] 19:12. https://doi.org/10.1186/s12940-020-0568-1
Gao P, Wu Y, He L et al (2022) Acute effects of ambient nitrogen oxides and interactions with temperature on cardiovascular mortality in Shenzhen, China. Chemosphere [j] 287:132255. https://doi.org/10.1016/j.chemosphere.2021.132255
Grudzińska EM, Zaborski D, Modrzejewska M (2022) Correlation between retrobulbar blood flow parameters and retinal nerve fiber, ganglion cell and inner plexus layer thickness in myopia. Eur J Ophthalmol [j] 32:643–650. https://doi.org/10.1177/1120672121992007
Grzybowski A, Kanclerz P, Tsubota K et al (2020) A review on the epidemiology of myopia in school children worldwide. BMC Ophthalmol [j] 20:27. https://doi.org/10.1186/s12886-019-1220-0
Guo J H, Dao X Y, Sun W Y (2021) An iron-nitrogen doped carbon and CdS hybrid catalytic system for efficient CO(2) photochemical reduction. Chem Commun (Camb) [J], 57: 2033–2036. doi:https://doi.org/10.1039/d0cc07692a
Han D, He MN, Zhu Y et al (2021) Protective effects of riboflavin-UVA-mediated posterior sclera collagen cross-linking in a guinea pig model of form-deprived myopia. Int J Ophthalmol [j] 14:333–340. https://doi.org/10.18240/ijo.2021.03.01
Hong J, Zhong T, Li H et al (2016) Ambient air pollution, weather changes, and outpatient visits for allergic conjunctivitis: a retrospective registry study. Sci Rep [j] 6:23858. https://doi.org/10.1038/srep23858
Hsu YA, Chen CS, Wang YC et al (2021) Anti-inflammatory effects of resveratrol on human retinal pigment cells and a myopia animal model. Curr Issues Mol Biol [j] 43:716–727. https://doi.org/10.3390/cimb43020052
Huang F, Shu Z, Huang Q et al (2022) Retinal dopamine D2 receptors participate in the development of myopia in mice. Invest Ophthalmol vis Sci [j] 63:24. https://doi.org/10.1167/iovs.63.1.24
Ikeda SI, Kurihara T, Toda M et al (2020) Oral bovine milk lactoferrin administration suppressed myopia development through matrix metalloproteinase 2 in a mouse model. Nutrients [j] 12. https://doi.org/10.3390/nu12123744
Jiang T, Gu J, Chen W et al (2019) Resveratrol inhibits high-glucose-induced inflammatory “metabolic memory” in human retinal vascular endothelial cells through SIRT1-dependent signaling. Can J Physiol Pharmacol [j] 97:1141–1151. https://doi.org/10.1139/cjpp-2019-0201
Jung SJ, Mehta JS, Tong L (2018) Effects of environment pollution on the ocular surface. Ocul Surf [j] 16:198–205. https://doi.org/10.1016/j.jtos.2018.03.001
Kalluri ROR, Gugamsetty B, Tandule CR et al (2021) Impact of aerosols on surface ozone during COVID-19 pandemic in southern India: a multi-instrumental approach from ground and satellite observations, and model simulations. J Atmos Sol Terr Phys [j] 212:105491. https://doi.org/10.1016/j.jastp.2020.105491
Kato M, Sato K, Habuta M et al (2019) Localization of the ultraviolet-sensor Opn5m and its effect on myopia-related gene expression in the late-embryonic chick eye. Biochem Biophys Rep [j] 19:100665. https://doi.org/10.1016/j.bbrep.2019.100665
Kearney S, O’Donoghue L, Pourshahidi LK et al (2019) Conjunctival ultraviolet autofluorescence area, but not intensity, is associated with myopia. Clin Exp Optom [j] 102:43–50. https://doi.org/10.1111/cxo.12825
Koch S, Zelembaba A, Tran R et al (2020) Vascular effects of physical activity are not modified by short-term inhaled diesel exhaust: results of a controlled human exposure study. Environ Res [j] 183:109270. https://doi.org/10.1016/j.envres.2020.109270
Korsiak J, Perepeluk KL, Peterson NG et al (2021) Air pollution and retinal vessel diameter and blood pressure in school-aged children in a region impacted by residential biomass burning. Sci Rep [j] 11:12790. https://doi.org/10.1038/s41598-021-92269-x
Kubota R, Joshi NR, Samandarova I et al (2021) Effect of short-term peripheral myopic defocus on ocular biometrics using Fresnel “press-on” lenses in humans. Sci Rep [j] 11:22690. https://doi.org/10.1038/s41598-021-02043-2
La Spina C, Corvi F, Bandello F et al (2016) Static characteristics and dynamic functionality of retinal vessels in longer eyes with or without pathologic myopia. Graefes Arch Clin Exp Ophthalmol [j] 254:827–834. https://doi.org/10.1007/s00417-015-3122-z
Lai L, Lv X, Wu X, et al. (2021) Comparing the differences in slowing myopia progression by riboflavin/ultraviolet A scleral cross-linking before and after lens-induced myopia in guinea pigs. Curr Eye Res [J] 1–9.doi:https://doi.org/10.1080/02713683.2021.2011324
Landis EG, Park HN, Chrenek M et al (2021) Ambient light regulates retinal dopamine signaling and myopia susceptibility. Invest Ophthalmol vis Sci [j] 62:28. https://doi.org/10.1167/iovs.62.1.28
Lasagni Vitar RM, Hvozda Arana AG, Janezic NS et al (2019) Urban air pollution induces redox imbalance and epithelium hyperplasia in mice cornea. Toxicol Appl Pharmacol [j] 384:114770. https://doi.org/10.1016/j.taap.2019.114770
Lee S S, Lingham G, Sanfilippo P G, et al. (2022) Incidence and progression of myopia in early adulthood. JAMA Ophthalmol [J]. doi:https://doi.org/10.1001/jamaophthalmol.2021.5067
Li X, Wu M, Zhang L et al (2017) Riboflavin and ultraviolet A irradiation for the prevention of progressive myopia in a guinea pig model. Exp Eye Res [j] 165:1–6. https://doi.org/10.1016/j.exer.2017.08.019
Li X, Zhang X, Zhang Z et al (2019) Air pollution exposure and immunological and systemic inflammatory alterations among schoolchildren in China. Sci Total Environ [j] 657:1304–1310. https://doi.org/10.1016/j.scitotenv.2018.12.153
Lin HJ, Wei CC, Chang CY et al (2016) Role of chronic inflammation in myopia progression: clinical evidence and experimental validation. EBioMedicine [j] 10:269–281. https://doi.org/10.1016/j.ebiom.2016.07.021
Lingham G, Mackey DA, Lucas R et al (2020) How does spending time outdoors protect against myopia? A review. Br J Ophthalmol [j] 104:593–599. https://doi.org/10.1136/bjophthalmol-2019-314675
Liu H, Gambino F Jr, Algenio CS et al (2020) Inflammation and oxidative stress induced by lipid peroxidation metabolite 4-hydroxynonenal in human corneal epithelial cells. Graefes Arch Clin Exp Ophthalmol [j] 258:1717–1725. https://doi.org/10.1007/s00417-020-04647-2
Liu Y, Wang L, Xu Y et al (2021) The influence of the choroid on the onset and development of myopia: from perspectives of choroidal thickness and blood flow. Acta Ophthalmol [j] 99:730–738. https://doi.org/10.1111/aos.14773
Louwies T, Panis LI, Kicinski M et al (2013) Retinal microvascular responses to short-term changes in particulate air pollution in healthy adults. Environ Health Perspect [j] 121:1011–1016. https://doi.org/10.1289/ehp.1205721
Louwies T, Nawrot T, Cox B et al (2015) Blood pressure changes in association with black carbon exposure in a panel of healthy adults are independent of retinal microcirculation. Environ Int [j] 75:81–86. https://doi.org/10.1016/j.envint.2014.11.006
Louwies T, Vuegen C, Panis LI et al (2016) miRNA expression profiles and retinal blood vessel calibers are associated with short-term particulate matter air pollution exposure. Environ Res [j] 147:24–31. https://doi.org/10.1016/j.envres.2016.01.027
Lu CW, Fu J, Liu XF et al (2021) Air pollution and meteorological conditions significantly contribute to the worsening of allergic conjunctivitis: a regional 20-city, 5-year study in Northeast China. Light Sci Appl [j] 10:190. https://doi.org/10.1038/s41377-021-00630-6
Luo H, Zhao K, Yuan Z et al (2021) Emission source-based ozone isopleth and isosurface diagrams and their significance in ozone pollution control strategies. J Environ Sci (China) [J] 105:138–149. https://doi.org/10.1016/j.jes.2020.12.033
Mahan VL (2020) Cardiac function dependence on carbon monoxide. Med Gas Res [j] 10:37–46. https://doi.org/10.4103/2045-9912.279982
Manisalidis I, Stavropoulou E, Stavropoulos A et al (2020) Environmental and health impacts of air pollution: a review. Front Public Health [j] 8:14. https://doi.org/10.3389/fpubh.2020.00014
Marchini T, Zirlik A, Wolf D (2020) Pathogenic role of air pollution particulate matter in cardiometabolic disease: evidence from mice and humans. Antioxid Redox Signal [j] 33:263–279. https://doi.org/10.1089/ars.2020.8096
Miyazaki D, Fukagawa K, Fukushima A et al (2019) Air pollution significantly associated with severe ocular allergic inflammatory diseases. Sci Rep [j] 9:18205. https://doi.org/10.1038/s41598-019-54841-4
Miyazaki D, Fukagawa K, Okamoto S et al (2020) Epidemiological aspects of allergic conjunctivitis. Allergol Int [j] 69:487–495. https://doi.org/10.1016/j.alit.2020.06.004
Montes CM, Demler HJ, Li S et al (2022) Approaches to investigate crop responses to ozone pollution: from O(3) -FACE to satellite-enabled modeling. Plant J [j] 109:432–446. https://doi.org/10.1111/tpj.15501
Morgan IG, Ohno-Matsui K, Saw SM (2012) Myopia. Lancet [j] 379:1739–1748. https://doi.org/10.1016/s0140-6736(12)60272-4
Morgan IG, French AN, Ashby RS et al (2018) The epidemics of myopia: aetiology and prevention. Prog Retin Eye Res [j] 62:134–149. https://doi.org/10.1016/j.preteyeres.2017.09.004
Mu JF, Zeng D, Yu SY et al (2020) Time-series analysis on the relationship between ambient PM2.5 and daily outpatient visits due to allergic conjunctivitis among children in Shenzhen. Zhonghua Yan Ke Za Zhi [j] 56:608–614. https://doi.org/10.3760/cma.j.cn112142-20191203-00623
Norton TT (2016) What do animal studies tell us about the mechanism of myopia-protection by light? Optom vis Sci [j] 93:1049–1051. https://doi.org/10.1097/opx.0000000000000917
Nucci P, Sacchi M, Pichi F et al (2017) Pediatric conjunctivitis and air pollution exposure: a prospective observational study. Semin Ophthalmol [j] 32:407–411. https://doi.org/10.3109/08820538.2015.1115088
Orru H, Ebi KL, Forsberg B (2017) The interplay of climate change and air pollution on health. Curr Environ Health Rep [j] 4:504–513. https://doi.org/10.1007/s40572-017-0168-6
Pan CW, Ramamurthy D, Saw SM (2012) Worldwide prevalence and risk factors for myopia. Ophthalmic Physiol Opt [j] 32:3–16. https://doi.org/10.1111/j.1475-1313.2011.00884.x
Provost EB, Int Panis L, Saenen ND et al (2017) Recent versus chronic fine particulate air pollution exposure as determinant of the retinal microvasculature in school children. Environ Res [j] 159:103–110. https://doi.org/10.1016/j.envres.2017.07.027
Rabito FA, Yang Q, Zhang H et al (2020) The association between short-term residential black carbon concentration on blood pressure in a general population sample. Indoor Air [j] 30:767–775. https://doi.org/10.1111/ina.12651
Riva M, Sun J, McNeill VF et al (2021) High pressure inside nanometer-sized particles influences the rate and products of chemical reactions. Environ Sci Technol [j] 55:7786–7793. https://doi.org/10.1021/acs.est.0c07386
Rong S, Wang C, Han B et al (2017) Iontophoresis-assisted accelerated riboflavin/ultraviolet A scleral cross-linking: a potential treatment for pathologic myopia. Exp Eye Res [j] 162:37–47. https://doi.org/10.1016/j.exer.2017.07.002
Rotolo M, Montani G, Martin R (2017) Myopia onset and role of peripheral refraction. Clin Optom (Auckl) [J] 9:105–111. https://doi.org/10.2147/opto.S134985
Ruan Z, Qian ZM, Guo Y et al (2019) Ambient fine particulate matter and ozone higher than certain thresholds associated with myopia in the elderly aged 50 years and above. Environ Res [j] 177:108581. https://doi.org/10.1016/j.envres.2019.108581
Schaeffel F (2017) Biological mechanisms of myopia. Ophthalmologe [j] 114:5–19. https://doi.org/10.1007/s00347-016-0388-4
Schikowski T (2022) Indoor and outdoor pollution as risk factor for allergic diseases of the skin and lungs. Handb Exp Pharmacol [j] 268:359–366. https://doi.org/10.1007/164_2021_503
Schraufnagel DE, Balmes JR, Cowl CT et al (2019) Air pollution and noncommunicable diseases: a review by the Forum of International Respiratory Societies’ Environmental Committee, Part 2: air pollution and organ systems. Chest [j] 155:417–426. https://doi.org/10.1016/j.chest.2018.10.041
Sendra VG, Tau J, Zapata G et al (2021) Polluted air exposure compromises corneal immunity and exacerbates inflammation in acute herpes simplex keratitis. Front Immunol [j] 12:618597. https://doi.org/10.3389/fimmu.2021.618597
Supharakonsakun Y, Areepong Y, Sukparungsee S (2020) The performance of a modified EWMA control chart for monitoring autocorrelated PM2.5 and carbon monoxide air pollution data. PeerJ 8:e10467. https://doi.org/10.7717/peerj.10467
Swiatczak B, Feldkaemper M, Schaeffel F (2019) Changes in fundus reflectivity during myopia development in chickens. Biomed Opt Express [j] 10:1822–1840. https://doi.org/10.1364/boe.10.001822
The L (2017) UK air pollution and public health. Lancet [j] 389:1860. https://doi.org/10.1016/s0140-6736(17)31271-0
Thomson K, Karouta C, Ashby R (2020a) Form-deprivation and lens-induced myopia are similarly affected by pharmacological manipulation of the dopaminergic system in chicks. Invest Ophthalmol vis Sci [j] 61:4. https://doi.org/10.1167/iovs.61.12.4
Thomson K, Morgan I, Karouta C et al (2020b) Levodopa inhibits the development of lens-induced myopia in chicks. Sci Rep [j] 10:13242. https://doi.org/10.1038/s41598-020-70271-z
United Nations Environment Programme (2016) Environmental effects of ozone depletion and its interactions with climate change: progress report, 2015. Photochem Photobiol Sci [J] 15:141–174. https://doi.org/10.1039/c6pp90004f
Wang Q, Deng Y, Li S et al (2021) Corneal biomechanical changes in allergic conjunctivitis. Eye Vis (Lond) [J] 8:17. https://doi.org/10.1186/s40662-021-00241-7
Wang Q, Yu F, Feng Z et al (2021) Changes in anterior and posterior corneal elevation in patients with allergic conjunctivitis. Front Med (Lausanne) [J] 8:788302. https://doi.org/10.3389/fmed.2021b.788302
Wang T, Li H, Zhang R et al (2021c) Evaluation of retinal vascular density and related factors in youth myopia without maculopathy using OCTA. Sci Rep [j] 11:15361. https://doi.org/10.1038/s41598-021-94909-8
Wei CC, Kung YJ, Chen CS et al (2018) Allergic conjunctivitis-induced retinal inflammation promotes myopia progression. EBioMedicine [j] 28:274–286. https://doi.org/10.1016/j.ebiom.2018.01.024
Wei CC, Lin HJ, Lim YP et al (2019) PM2.5 and NOx exposure promote myopia: clinical evidence and experimental proof. Environ Pollut [J] 254:113031. https://doi.org/10.1016/j.envpol.2019.113031
Williams KM, Bentham GC, Young IS et al (2017) Association between myopia, ultraviolet B radiation exposure, serum vitamin D concentrations, and genetic polymorphisms in vitamin D metabolic pathways in a multicountry European study. JAMA Ophthalmol [j] 135:47–53. https://doi.org/10.1001/jamaophthalmol.2016.4752
Wirz-Justice A, Skene DJ, Münch M (2021) The relevance of daylight for humans. Biochem Pharmacol [j] 191:114304. https://doi.org/10.1016/j.bcp.2020.114304
Witters K, Dockx Y, Op’tRoodt J et al (2021) Dynamics of skin microvascular blood flow in 4–6-year-old children in association with pre- and postnatal black carbon and particulate air pollution exposure. Environ Int [J] 157:106799. https://doi.org/10.1016/j.envint.2021.106799
Wolkoff P (2017) External eye symptoms in indoor environments. Indoor Air [j] 27:246–260. https://doi.org/10.1111/ina.12322
Wu PC, Chuang MN, Choi J et al (2019) Update in myopia and treatment strategy of atropine use in myopia control. Eye (Lond) [J] 33:3–13. https://doi.org/10.1038/s41433-018-0139-7
Xin Y, Kang BS, Zheng YP et al (2021) Biophysical properties of corneal cells reflect high myopia progression. Biophys J [j] 120:3498–3507. https://doi.org/10.1016/j.bpj.2021.05.010
Xu D, Yuan Z, Wang M et al (2022) Multi-factor reconciliation of discrepancies in ozone-precursor sensitivity retrieved from observation- and emission-based models. Environ Int [j] 158:106952. https://doi.org/10.1016/j.envint.2021.106952
Yang BY, Guo Y, Zou Z et al (2021) Exposure to ambient air pollution and visual impairment in children: a nationwide cross-sectional study in China. J Hazard Mater [j] 407. https://doi.org/10.1016/j.jhazmat.2020.124750
Ying G, Tang Z, Zhang J, et al. (2021) Long noncoding RNA CASC2 protect ROS-induced oxidative stress in myocardial infarction by miR-18a/SIRT2. Biotechnol Appl Biochem [J]. doi:https://doi.org/10.1002/bab.2252
Yuan J, Wu S, Wang Y et al (2019) Inflammatory cytokines in highly myopic eyes. Sci Rep [j] 9:3517. https://doi.org/10.1038/s41598-019-39652-x
Zhang J, Deng G (2020) Protective effects of increased outdoor time against myopia: a review. J Int Med Res [j] 48:300060519893866. https://doi.org/10.1177/0300060519893866
Zhang Q, Jan C, Guo CY et al (2018) Association of intraocular pressure-related factors and retinal vessel diameter with optic disc rim area in subjects with and without primary open angle glaucoma. Clin Exp Ophthalmol [j] 46:389–399. https://doi.org/10.1111/ceo.13042
Zheng Q, Zong Y, Li L et al (2015) Retinal vessel oxygen saturation and vessel diameter in high myopia. Ophthalmic Physiol Opt [j] 35:562–569. https://doi.org/10.1111/opo.12223
Zhou X, Pardue MT, Iuvone PM et al (2017) Dopamine signaling and myopia development: what are the key challenges. Prog Retin Eye Res [j] 61:60–71. https://doi.org/10.1016/j.preteyeres.2017.06.003
Funding
This work was supported by Chinese National Key Research and Development Program (Project number 2021YFC2702100); Chinese National Nature Science Foundation (Project number 82071012); The Science and Technology Commission of Shanghai Municipality (Project No. 20DZ1100200); Shanghai Municipal Commission of Health (Public Health System Three-Year Plan-Key Subjects) (Project No. GWV10.1-XK7); The Project of Shanghai Shen Kang Hospital Development Centre (Grant Nos. SHDC2020CR30538, SHDC2018110); Shanghai Engineering Research Center of Precise Diagnosis and Treatment of Eye Diseases, Shanghai, China (Project No. 19DZ2250100); and Shanghai General Hospital, Clinical Research CTCCR-2018Z01.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. T. Y. made the tables and figure and wrote the first draft of the manuscript. H. Z. reviewed the manuscript and designed the figure. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
No ethical issue is to be declared in this article.
Consent to participate
No consent of participation is to be claimed.
Consent for publication
All of the authors have read and approved the paper for publication. We confirmed that it has not been published previously nor is it being considered by any other peer-reviewed journal.
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Lotfi Aleya
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yuan, T., Zou, H. Effects of air pollution on myopia: an update on clinical evidence and biological mechanisms. Environ Sci Pollut Res 29, 70674–70685 (2022). https://doi.org/10.1007/s11356-022-22764-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-22764-9