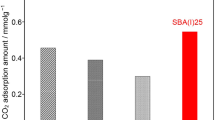
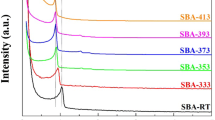
Abstract
The present work reports the changes for the mesoporous materials SBA-15 and KIT-6 associated with the structural, textural, and chemical properties when they are subjected to thermo-alkaline treatment. Despite the fact that the silica supports have not a strong affinity for CO2 adsorption, the adsorption enthalpy profiles (ΔHads) reported that the substrates subjected to the thermo-alkaline treatment (S15H and K6H) have a greater energetic affinity towards CO2 capture if compared to the precursory solids (S15 and K6). The ΔHads is − 26.7 kJ mol−1 at 0.15 mmol g−1 by supported S15H and K6H while the ΔHads is − 20. 7 kJ mol−1 and − 18.7 kJ mol−1 by K6 and S15, respectively, at the same CO2 coverage. Furthermore, the CO2 adsorption performances by the hydrolytic condensation between silica supports and the N´- (3-trimethoxysilylpropyl)diethylenetriamine (NAEPTES) or 3-aminopropiltriethoxysilane (APTES) are presented and it can be seen that the best performer for CO2 adsorption is reported for the S15HN since it is able to absorb 0.93 mmol at 0.15 atm at 318 K. Thereby, the outcomes show that the effects of porous curvature and the magnitude of the amine species are parameters to be considered, as well as the thermo-alkaline treatment, in order to improve the subsequent surface reactions on silica supports. The materials were characterized by XRD, TEM, and N2 adsorption at 77 K, NIR, and pyridine thermodesorption using Fourier Transform Infrared Spectroscopy (FTIR-Py), NMR for 29Si and 13C, DSC, and CO2 adsorption.
Graphical abstract











modified by the presence of NAEPTES: S15N, S15HN, and K6HN



Similar content being viewed by others
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Babaei M, Salehi S, Anbia M, Kazemipour M (2018) Improving CO2 adsorption capacity and CO2/CH4 selectivity with amine functionalization of MIL-100 and MIL-101. J Chem Eng 63(5):1657–1662
Bal R, Tope BB, Das TK, Hegde SG, Sivasanker S (2001) Alkali-loaded silica, a solid base: investigation by FTIR spectroscopy of adsorbed CO2 and its catalytic activity. J Catal 204(2):358–363
Belmabkhout Y, Sayari A (2009) Effect of pore expansion and amine functionalization of mesoporous silica on CO2 adsorption over a wide range of conditions. Adsorption 15:318–328
Canivet J, Fateeva A, Guo Y, Coasne B, Farrusseng D (2014) Water adsorption in MOFs: fundamentals and applications. Chem Soc Rev 43(16):5594–5617
Coenen KT, Gallucci F, Cobden P, van Dijk E, Hensen EJM, van Sint AM (2017) Chemisorption of H2O and CO2 on hydrotalcites for sorptionenhanced water-gas-shift processes. Energy Procedia 114:2228–2242
Garcés-Polo SI, Villarroel-Rocha J, Sapag K, Korili SA, Gil A (2018) Adsorption of CO2 on mixed oxides derived from hydrotalcites at several temperatures and high pressures. Chem Eng J 332:24–32
Garshasbi V, Jahangiri M, Anbia M (2017) Equilibrium CO2 adsorption on zeolite 13X prepared from natural clays. Appl Surf Sci 393:225–233
Hakim A, Marliza TS, Abu Tahari NM et al (2016) Studies on CO2 adsorption and desorption properties from various types of iron oxides (FeO, Fe2O3, and Fe3O4). Ind Eng Chem Res 55(29):7888–7897
Hudson S, Tanner DA, Redington W et al (2006) Quantitative TEM analysis of a hexagonal mesoporous silicate structure. Phys Chem Chem Phys 8(29):3467–3474
Iqbal N, Wang X, Yu J, Ding B (2017) Robust and flexible carbon nanofibers doped with amine functionalized carbon nanotubes for efficient CO2 capture. Adv Sustainable Syst 1(3–4):1600028
Karthikeyan G, Pandurangan A (2012) Post synthesis alumination of KIT-6 materials with Ia3d symmetry and their catalytic efficiency towards multicomponent synthesis of 1H-Pyrazolo[1,2-] Phthalazine-5,10-dione carbonitriles and carboxylates. J Mol Catal a: Chem 361–362:58–67
Lee SH, Kang JS, Kim D (2018) A mini review: recent advances in surface modification of porous silicon. Materials 11(12):2557
Lee ZH, Lee KT, Bhatia S, Mohamed AR (2012) Post-combustion carbon dioxide capture: evolution towards utilization of nanomaterials. Renew Sustain Energy Rev 16(5):2599–2609
López T, Basaldella EI, Ojeda ML, Manjarrez J, Alexander-Katz R (2006) Encapsulation of valproic acid and sodic phenytoin in ordered mesoporous SiO2 solids for the treatment of temporal lobe epilepsy. Opt Mater 29(1):75–81
Medina-Juárez O, García-Sánchez MÁ, Arellano-Sánchez U, Kornhauser-Straus I, Rojas-González F (2016) Optimal surface amino-functionalization following thermo-alkaline treatment of nanostructured silica adsorbents for enhanced CO2 adsorption. Materials 9(11):898
Ojeda-López R, Pérez-Hermosillo IJ, Esparza-Schulz JM, Cervantes-Uribe A, Domínguez-Ortiz A (2015) SBA-15 materials: calcination temperature influence on textural properties and total silanol ratio. Adsorption 21(8):659–669
Pal A, Uddin K, Rocky KA, Thu K, Saha BB (2019) CO2 adsorption onto activated carbon–graphene composite for cooling applications. Int J Refrig 106:558–569
Perry CC, Li X (1991) Structural studies of gel phases part 1. -Infrared spectroscopic study of silica monoliths; the effect of thermal history on structure. J Chem Soc Faraday Trans 87:761–766
Pham TD, Hudson MR, Brown CM, Lobo RF (2014) Molecular basis for the high CO2 adsorption capacity of chabazite zeolites. Chemsuschem 7(11):3031–3038
Pinto L, Mafra L, Guil JM, Pires J, Rocha J (2011) Adsorption and activation of CO2 by amine-modified nanoporous materials studied by solid-state NMR and 13CO2 adsorption. Chem Mater 23:1387–1395
Sanz-Pérez ES, Dantas TCM, Arencibia A et al (2017) Reuse and recycling of amine-functionalized silica materials for CO2 adsorption. Chem Eng J 308:1021–1033
Songolzadeh M, Soleimani M, Ravanchi MT, Songolzadeh R (2014) Carbon dioxide separation from flue gases: a technological review emphasizing reduction in greenhouse gas emissions. Sci World J 2014(828131):34
Thommes M, Kaneko K, Neimark AV et al (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl Chem 87(9–10):1051–1069
UN Global Compact, and Duke University (2010) Environmental stewardship strategy - overview and resource for corporate leaders. Published by the UN Global Compact, and Duke University. https://www.unglobalcompact.org/library/331. Accessed June 2010
Van Der Voort P, Gills-D’Hamers I, Vansant EF (1990) Estimation of the distribution of surface hydroxyl groups on silica gel, using chemical modification with trichlorosilane. J Chem Soc Faraday Trans 86(22):3751–3755
Wang S, Yan S, Ma X, Gong J (2011) Recent advances in capture of carbon dioxide using alkali-metal-based oxides. Energy Environ Sci 4(10):3805–3819
Wang W, Qi R, Shan W et al (2014) Synthesis of KIT-6 type mesoporous silicas with tunable pore sizes, wall thickness and particle sizes via the partitioned cooperative self-assembly process. Micropor Mesopor Mat 194:167–173
Weimin Z, Chishti MZ (2021) Toward sustainable development: assessing the effects of commercial policies on consumption and production-based carbon emissions in developing economies. SAGE Open 11(4):1–18.
Zakharova MV, Masoumifard N, Hu Y et al (2018) Designed synthesis of mesoporous solid-supported Lewis acid-base pairs and their CO2 adsorption behaviors. ACS Appl Mater Interfaces 10(15):13199–13210
Zaki MI, Hasan MA, Al-Sagheer FA, Pasupulety L (2001) In situ FTIR spectra of pyridine adsorbed on SiO2–Al2O3, TiO2, ZrO2 and CeO2: general considerations for the identification of acid sites on surfaces of finely divided metal oxides. Colloids Surf, A 190:261–274
Zhao D, Feng J, Huo Q et al (1998) Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 279(5350):548–552
Acknowledgements
The authors gratefully acknowledge Dr. Federico Gonzalez García of the DRX laboratory (INFR-2011-1-163250), Dra. Gloria del Angel Montes (FTIR-Py), Ing. Patricia Castillo (SEM/TEM), and M.C. Marco A. Vera (RMN).
Funding
This research was funded by the Consejo Nacional de Ciencia y Tecnología (CONACyT) and the projects “Physical Chemistry of Surfaces” (UAM-I CA-031) and the Academic Network “Nanoscopic and Textural Design of Advanced Materials.” OMJ thanks CONACyT for the support with the scholarship (442345).
Author information
Authors and Affiliations
Contributions
O.M-J.: writing – original draft, methodology, investigation, and formal analysis. I.R-V. and R.O-L: formal analysis and investigation. M.A.S-G. and F.R-G.: supervision, writing – review and editing, and resources.
Corresponding author
Ethics declarations
Ethics approval
All authors affirm that objectivity and transparency in research have been ensured and ensure that accepted principles of ethical and professional conduct have been followed.
Consent for publication
All authors agree with the content and have given their explicit consent to submit the paper.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: George Z. Kyzas
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Medina-Juárez, O., Rangel-Vázquez, I., Ojeda-López, R. et al. Importance of the polarity on nanostructured silica materials to optimize the hydrolytic condensation with molecules related to CO2 adsorption. Environ Sci Pollut Res 29, 58472–58483 (2022). https://doi.org/10.1007/s11356-022-21540-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-21540-z