Abstract
Amine-grafted adsorbents are promising CO2 adsorbents; however, the excessive addition of an amino silane coupling agent during their synthesis increases their production cost. Thus, using low amounts of silane, we synthesized 3-aminopropyltrimethoxysilane (APTMS)-grafted SBA-15 mesoporous silica and evaluated its CO2 adsorption performance. APTMS-grafted SBA-15 samples were prepared using either impregnation or heating–filtration method (grafting). The obtained samples were characterized by X-ray diffraction spectroscopy, transmission electron microscopy, N2 adsorption/desorption, scanning electron microscopy, magic-angle spinning nuclear magnetic resonance, and elemental analysis. The results revealed that the micropores of SBA-15 were preferentially blocked, and APTMS increasingly occupied the mesopores with increasing amine loading. The CO2-adsorption performance of the adsorbents was measured by thermogravimetric analysis under dry conditions. Both synthesis methods achieved high amine immobilization efficiency (78.3–92.2%), as estimated from the amount of silane coupling agents used in the synthesis and that immobilized on the support. The adsorbents prepared by the two methods adsorbed similar amounts of CO2 of approximately 0.5 mmol g− 1 in 400 ppm CO2 and ~ 1.0 mmol g− 1 in 5 vol% CO2. The adsorption amounts attained in this study are comparable to those of previously reported silane-coupling-agent-modified adsorbents that were prepared with more silane. In contrast, the adsorption rate of the samples was affected by the synthesis method, even with similar amine loadings. Nonetheless, the results revealed that even with a low amount of the silane coupling agent, high-performance amine-grafted CO2 adsorbents could be synthesized.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The emission of CO2 is considered as a primary contributor to global warming [1,2,3,4,5]. Carbon capture and storage (CCS) and carbon capture and utilization (CCU) are pivotal technologies for addressing the challenges posed by the greenhouse effect of CO2 [6,7,8,9], with CO2 capture being the initial step in both technologies [8]. Although amine scrubbing is the conventional method used to eliminate CO2 from industrial waste gases, it does have certain drawbacks [10, 11], including high energy consumption, susceptibility to amine decomposition in the absorption solution, and severe equipment corrosion [12,13,14]. In addition, regenerating amine solvents requires a substantial amount of heat [15]. This regeneration heat comprises three components: adsorption heat for the CO2-stripping reaction, sensible heat for temperature elevation, and vaporization heat for converting liquid water to vapor for CO2 stripping [16].
In light of these drawbacks, amine-based solid adsorbents consisting of amine molecules or functional groups within solid materials have garnered significant attention [17]. Porous solids can reduce the energy required for regeneration owing to their inherently lower heat capacities compared with those of water and liquid adsorbents [18]. Furthermore, this type of adsorbent offers great advantages in terms of equipment corrosion and fast adsorption kinetics [17, 19].
Based on their preparation methods, amine-based solid adsorbents can be categorized into Classes I, II, and III. Class I adsorbents are created using the impregnation method, wherein an amine is loaded onto the supports and retained through van der Waals forces. Class II adsorbents are prepared by chemical grafting, which combines amine components (such as silane coupling agents) with supports via condensation. Finally, Class III adsorbents have been prepared through in situ polymerization [20, 21]. Class I adsorbents generally possess high amine loadings and consequently a high CO2 capture capacity. However, they tend to have relatively low stabilities and adsorption rates [17, 22,23,24]. The CO2 adsorption capacities of Class II adsorbents are typically lower than those of Class I (i.e., amine-impregnated) adsorbents owing to their lower nitrogen content. Nonetheless, Class II adsorbents exhibit higher durability during adsorption/desorption cycling because of the presence of covalent bonds [25,26,27,28,29], which are more stable than van der Waals forces.
Numerous Class II adsorbents have been developed to date [30,31,32,33]. For instance, 2-[2-(3-trimethoxysilylpropylamino)ethylamino]ethylamine-grafted silica aerogels [25] could maintain an adsorption amount of 2.3 mmol g− 1 for 100 adsorption cycles in 1 bar of CO2. Meanwhile, SBA-12 grafted with 3-aminopropyltriethoxysilane [34] exhibited a capacity of 1.5 mmol g− 1 at 25 °C under a flow of 10 vol% CO2. Chang et al. [35] synthesized SBA-15 modified with (3-trimethoxysilylpropyl)diethylenetriamine, achieving a high adsorption amount of 2.74 mmol g− 1 at 40 °C under a dry 17 vol% CO2 gas stream. Surprisingly, however, reducing the use of expensive aminosilane has received little attention in the research on Class II adsorbents.
The essential parameters for CO2 capture include the adsorption performance, such as the adsorption amount, adsorption rate, and stability. However, practical applications also require consideration of the cost of the adsorbents. Hasmukh et al. [36] emphasized the importance of atomic economy in adsorbent preparation when scaling up for industrial waste gas treatment. However, existing approaches for synthesizing Class II adsorbents require excessive amounts of aminosilane. For instance, in previous studies employing 2-[2-(3-trimethoxysilylpropylamino)ethylamino] [37], (3-aminopropyl)trimethoxysilane (APTMS) [38], and (3-aminopropyl)triethoxysilane (APTES) [39], the synthesis systems immobilized only 7.3%, 15%, and 61% of the total silane used therein, respectively. In addition, amino silane is typically more expensive than other reagents, such as porous supports and solvents. Thus, recent research on the synthesis of Class II materials has evidently failed to meet both the requirements of atom economy and low costs. Linneen et al. [25] prepared amine-grafted silica aerogel adsorbents with various amine/silica ratios (from 0.5:1 to 4:1 gamine:gsilica). However, the impact of the quantity of added silane, especially in the low range, remains unclear. At present, no studies have focused on reducing the amount of aminosilanes used to synthesize Class II adsorbents. Therefore, in this research, we aimed to prepare a Class II adsorbent with reduced amounts of a silane coupling agent, characterize the resulting materials, and investigate their CO2 adsorption performance. In the typical preparation process for amine-grafted adsorbents, aminosilane, and a porous support are mixed in an organic solvent. Subsequently, the mixture is heated and stirred for a specified duration. Finally, the solvent is removed through filtration, along with any excess aminosilane. This procedure is commonly known as the grafting method.
Ribeiro et al. [40] suggested evaporation as a cost-effective approach for solvent recovery and reuse. However, they reported that the Class II adsorbent prepared using the evaporation method did not demonstrate satisfactory performance, but their investigation of the amount of silane was limited to only one experimental condition. In light of this perspective, we also investigated synthesis using the evaporation method, which we refer to as impregnation herein. Reduced the amount of silane coupling agent brings can improve the atomic utilization ratio of aminosilane-modified silica adsorbents, and the research outcome obtained here would contribute to the large-scale production of Class II adsorbents and the corresponding practical CCUS process. In this study, we synthesized APTMS-modified SBA-15 using two different methods: impregnation and heating–filtration. These methods are commonly employed for modifying mesoporous silica, with the key reagents being APTMS and APTES [41].
SBA-15 has been extensively researched as a support material for Class II adsorbents. Mesoporous silica SBA-15 was first reported by Zhao et al. in 1998 [42]. It possesses a well-defined, large, and uniform 1D mesoporous structure with micropores on the silica walls [43]. The introduced silane readily diffuses to create adsorption sites within the mesopores.
We initially focused on optimizing the choice of solvent used for the synthesis and determining the appropriate quantity of amine to be added. Then, to investigate the impact of amine addition on the silylation behavior, we characterized the structures of the samples before and after adding amine using X-ray diffraction (XRD), N2 adsorption/desorption, transmission electron microscopy (TEM), magic-angle spinning (MAS) nuclear magnetic resonance (NMR) spectroscopy, scanning electron microscopy (SEM), and CHN analysis.
The CO2 adsorption experiments were conducted at a CO2 concentration of 400 ppm and 5 vol% CO2 gas flow. 400 ppm was chosen to simulate the conditions for direct air capture (DAC), wherein CO2 is captured from the atmosphere at low concentrations of approximately 400 ppm [44].
2 Materials and methods
Pluronic P123 (EO20PO70EO20, Sigma-Aldrich), tetraethyl orthosilicate (TEOS, Tokyo Chemical Co. Inc.), hydrochloric acid (HCl, 36 wt%, Fujifilm Wako Chemical Co.), toluene (99.5%, super dehydrated, Fujifilm Wako Chemical Co.), acetonitrile (99.8%, super dehydrated, Fujifilm Wako Chemical Co.), ethanol (99.5%, super dehydrated, Fujifilm Wako Chemical Co.), methanol (99.8%, super dehydrated, Fujifilm Wako Chemical Co.), and APTMS (96%, Tokyo Chemical Co. Inc.) were used as received.
2.1 Synthesis of SBA-15
SBA-15 was synthesized based on procedures in previous studies [42, 45]. First, 20 g of Pluronic P123 was added to 525 g of deionized water and stirred at 30 °C until it was completely dissolved. Then, 42.63 g of TEOS and 148.2 g of HCl were introduced to the mixture, which was stirred for an additional 20 h on a magnetic stirrer while heating at 30 °C. Subsequently, the reaction vessel was transferred to an oil bath and stirred under reflux for another 20 h. The resulting gel was then filtered and rinsed with water, and the obtained solid was dried at 70 °C overnight. Finally, to eliminate the organic components, the product was calcinated in air at 550 °C for 8 h.
2.2 Adsorbent preparation
2.2.1 Impregnation method
SBA-15 (0.3 g) was dispersed in 5 ml of toluene, methanol, ethanol, or acetonitrile each containing a specific quantity of APTMS (20, 25, 30, and 40 wt%). These mixtures were separately placed in a 10-ml glass bottle. Subsequently, the mixture was subjected to drying at 70 °C for 24 h to completely eliminate the solvent. Through this solvent screening process, acetonitrile was determined to be the most suitable solvent for preparing samples through both the impregnation and heating–filtration methods.
APTMS was loaded onto SBA-15 via the condensation reaction shown in Eq. (1), and the weight of the added amine was calculated using Eq. (2), considering the removal of methanol.
where MAPTMS, MCH3O–, and MMeOH correspond to the masses of APTMS, CH3O– in APTMS, and methanol, respectively. It was assumed that all methoxy groups from APTMS were converted into methanol. Amine addition is the amount of amino silane added during the synthesis process. The samples produced through the impregnation method are hereafter denoted as SBA(I)x, where x represents the weight% of the introduced amine, e.g., SBA(I)25 was prepared through impregnation with 25 wt% amine.
2.2.2 Heating–filtration method
SBA-15 (0.3 g) and a specific quantity of APTMS (15, 20, 25, 30, 40, 100, and 250 wt%) were introduced into a 10-ml glass bottle containing 5 ml of acetonitrile. The mixture was stirred for 1 d at 70 °C. Subsequently, the product was filtered and rinsed with 40 ml of acetonitrile. The resulting solid product was dried at 70 °C overnight.
The samples prepared through the heating–filtration methods are hereafter denoted as SBA(HF)x, where x represents the weight% of amine. For instance, SBA(HF)30 was prepared using the heating–filtration method with 30 wt% amine.
2.3 Characterization of adsorbents
The crystalline structures of the samples were determined using low-angle XRD (Bruker PHASER) with Cu Kα radiation at 30 kV and a tube current of 10 mA. N2-adsorption isotherms were obtained using a BELSORP-max instrument (Bel, Japan). Prior to measurement, the samples were pretreated at 50 °C under vacuum for 3 h. N2-adsorption/desorption isotherms were conducted at − 196 °C. The surface area was calculated using the Brunauer–Emmett–Teller (BET) method, and the pore size distribution was estimated using the Barrett–Joyner–Halenda (BJH) method with N2 adsorption isotherm. The micropore volumes of the samples were calculated using the t-plot method. The sample morphology was observed using SEM with a Hitachi S-4800 microscope. The TEM images were acquired using a JEOL JEM-2100 microscope at an accelerating voltage of 200 kV. 29Si and 13C NMR spectra were measured using a Varian 600PS solid NMR spectrometer with a 5 mm diameter zirconia rotor spinning at 7 kHz. The actual amine loadings, which is the actual amine content loaded in SBA-15, were determined using a CHN analyzer (PerkinElmer 2400II) at the Advanced Science Research Center, Okayama University.
2.4 CO2 adsorption
CO2 adsorption under dry conditions was performed using a thermogravimetric (TG) instrument (STA200, Hitachi). Approximately 5 mg of the sample was placed in an aluminum container and pre-treated at 80 °C under a pure N2 flow of 500 ml min–1 until the weight stabilized. Subsequently, the temperature was reduced to 30 °C under the same N2 flow, and this cooling phase lasted for an additional 0.5 h, following which the samples were exposed to a CO2 flow of 400 ml min–1 (containing either 400 ppm or 5 vol% of CO2 balanced with N2) for 60 min.
Recycling experiments for CO2 adsorption/desorption were also performed under similar conditions by repeated cycles of heating at 80 °C and adsorption at 30 °C. The weight variation over time in the recycling experiments is schematically illustrated in Fig. S1. The CO2 adsorption quantities of the adsorbents were determined using Eq. (3) as follows:
where mCO2 adsorption is the weight of adsorbed CO2, madsorbent is the weight of the adsorbent, and mCO2 desorption is the weight loss of the adsorbent after heating at 80 °C.
The adsorption rate (mmol g–1 min–1) was defined as the CO2 adsorption amount per unit weight of adsorbent per minute and was calculated from the slope of the TG curves.
3 Results and discussion
Ribeiro et al. [40] reported that the choice of solvent plays a pivotal role in the grafting of silane coupling agents. Jaksa et al. [46] studied the impact of various solvents on the state of APTMS-modified silicon surfaces. Their results revealed that the size of the APTMS polymer on silicon and the morphology of the modified surface were both highly dependent on the type of solvent employed. Consequently, we employed a variety of solvents to create adsorbent samples through the impregnation method and assessed the CO2 adsorption capacity of these samples under a dry 400 ppm CO2 stream using TG. The amount of amine added to the samples was set to 25 wt%.
The solvents used for the synthesis were categorized into three types: polar protic solvents (ethanol and methanol), polar aprotic solvents (acetonitrile), and nonpolar solvents (toluene). As depicted in Fig. 1, the sample prepared with acetonitrile exhibited the largest CO2 adsorption capacity, measuring at 0.546 mmol g− 1. We therefore proceeded to prepare the materials using acetonitrile as the solvent. As Jaksa et al. [46] mentioned, acetonitrile is more conducive to the deposition of APTMS on the silicon surface and leads to the formation of a rough layer with a large number of APTMS polymer islands. At present, although the immobilization behavior of APTMS is not entirely clear, we expect that property of solvent such as polarity and affinity have an important role to effectively immobilizing APTMS on the SBA-15 in this study.
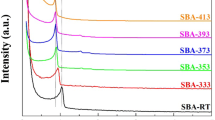
Acetonitrile was employed as the solvent to prepare amine-loaded SBA-15 adsorbents using different amounts of amine through either impregnation or heating–filtration, which are referred to as I and HF in all sample labels hereafter. Figure 2 displays the XRD patterns of SBA-15, SBA(I)25, and SBA(HF)30, and Fig. S2 presents those of the rest of the SBA(I) and SBA(HF) samples with various amounts of APTMS. SBA-15 exhibits diffraction peaks at 2θ = 0.9°, 1.7°, and 1.9°, attributed to the 100, 110, and 200 planes in the hexagonal structure of mesoporous silica SBA-15 [47, 48]. However, following APTMS grafting, the diffraction peaks at 2θ = 1.7° and 1.9° vanished, as shown in Figs. 2 and S2, indicating slight structural disorder due to the integrated silane coupling agent [49,50,51]. In the XRD patterns of both the SBA(I) and SBA(HF), the intensity of the diffraction peak at 0.9° decreases with the increasing amount of APTMS. In other studies on aminosilane-coupling-agent-modified silica materials [52,53,54], similar phenomena were observed. After amine modification, the surface of SBA-15 became occupied by APTMS, which reduced the diffraction peak intensity owing to a decrease in the scattering contrast caused by the organic groups attached to the surface of the support [55].
Figure 3 shows the N2 adsorption/desorption isotherms and pore distributions of the samples, and the textural parameters are listed in Table 1. The isotherms of SBA-15, SBA(I)25, and SBA(HF)30 exhibited Type IV behavior accompanied by H1 hysteresis loops [56]. This pattern indicates that these materials possessed highly ordered mesoporous structures with narrow pore-size distributions [48, 57]. In the case of pure SBA-15, the isotherm revealed a distinctive sharp inflection at P/P0 = 0.5–0.7, indicating the capillary condensation of adsorbed N2 within uniformly sized pores [58]. The position of this inflection correlated with the mesopore diameter [59]. Notably, the inflections of SBA(I)25 and SBA(HF)30 shifted to lower relative pressures, indicating smaller pores. Furthermore, the pore size distributions of the samples confirmed that the APTMS modification decreased the mesopore diameter. As shown in Table 1, the incorporation of APTMS reduced the specific surface area, pore volume, and mesopore diameter of the samples.
Figure 4 illustrates the relationship between amine addition and the surface area of the adsorbents. The surface areas of the samples prepared using both methods decreased as the APTMS content increased. When it reached 30 wt% in SBA(I)30, the specific surface area of SBA-15(I) samples approached zero. This substantial decrease in the specific surface area was attributed to pore blocking resulting from the excessive addition of APTMS, as evidenced by the N2 adsorption/desorption characterization data (Fig. S3). The degree of pore blocking in the SBA(HF) samples also increased with the increasing amine content.
In contrast to the SBA(I) samples, the specific surface area of the SBA(HF) samples did not significantly reduce, even when the APTMS content exceeded 30 wt%. For instance, SBA(HF)30 and SBA(HF)40 exhibited specific surface areas of 140 and 73 m2 g− 1, respectively. This difference can be attributed to the filtration step during the synthesis process, which removed excess APTMS that did not react with SBA-15 from the reaction system. Figure 4 shows that even with higher APTMS contents, the SBA(HF) samples maintained a certain surface area. Specifically, SBA(HF)100 and SBA(HF)250 had specific surface areas of 151 and 216 m2 g− 1, respectively, which could also be attributed to the removal of excess APTMS. We also compared the specific surface areas of our samples to those of previously reported amine-grafted silica adsorbents prepared with larger amounts of amine [35, 60,61,62,63], and we found they were consistent, as shown in Fig. 4.
SBA-15 exhibits two distinct pore structures: mesopores (5–30 nm) [64] and micropores (0.6–2 nm) [65]. To examine the silane loading behavior within SBA-15, we determined the remaining fractions of the micropore and mesopore volumes as percentages. The micropore and mesopore volumes of the samples were estimated using the t-plot method, and the percentages of the remaining volumes of mesopores and micropores were calculated using Eqs. (4) and (5), respectively.
where \({V}_{SBA-15}^{meso}\) and \({V}_{sample}^{meso}\) are the mesopore volumes of the pure and modified SBA-15 adsorbents, respectively; \({V}_{SBA-15}^{micro}\)and \({V}_{sample}^{micro}\) are the corresponding micropore volumes; and \({wt}_{SBA-15}\) is the mass fraction of SBA-15 in the adsorbent.
Figure 5 shows the fraction of the remaining pore volume in the pure and APTMS-modified SBA-15 adsorbents. Even at the lowest amount of added amine (i.e., 15 wt%), the micropore volume of SBA-15 significantly reduced to nearly zero for both methods, indicating the preferential blocking of the micropores in SBA-15. The fraction of the remaining mesopore volume in the SBA(I) samples almost reached zero when the amine addition exceeded 30 wt%. By contrast, in the SBA(HF) samples, this fraction gradually decreased with the increasing amine content but never reached zero; thus, SBA(HF) retained some mesopore volume, even at higher amine concentrations (100 and 250 wt%).
Figure 6 shows the average pore diameters of adsorbents with various amine concentrations, and Fig. S4 shows the corresponding pore size distributions. The average pore diameters of both the SBA(I) and SBA(HF) samples decreased as the amount of added APTMS increased within the range of 0–30 wt%.
Based on the texture data of samples, although a large amount of APTMS was added during the preparation of samples SBA(HF) 250, its specific surface area, pore volume, and pore size were higher than those of SBA(HF)100. As mentioned earlier, SBA(HF)100 and SBA(HF)250 were prepared by heating-filtration method, in the presence of excessive APTMS. However, excessive APTMS can be removed by the subsequent filtration protocol on these syntheses. Yannick et al [66] pointed out that, in the process of amino silane grafting, amino silane will preferentially undergo intramolecular condensation reaction rather than reacting with hydroxyl groups on the silicon surface in their report. In Jaksa et al. [46] report, they used APTMS to modify silicon wafers and found the size of condensed APTMS polymer on silicon can reach 10 nm. High amino silane addition can promote intramolecular condensation and form larger APTMS polymer. Therefore, the large APTMS polymer formed on the large amount of APTMS addition is possibly removed by washing because it cannot be incorporated into the mesopore of SBA-15. As a result, only a portion of APTMS can be loaded in SBA-15.
In the SEM images of SBA(I)30 and SBA(I)40, which have small specific surface areas (Fig. S5), large particles with sizes exceeding 5 μm are observed alongside the modified SBA-15 particles. Similarly, an SEM image of the APTMS polymer obtained by drying a mixture of acetonitrile solutions (Fig. S6) exhibits comparable irregular morphological structures. Thus, the presence of these particles in SBA(I)30 and SBA(I)40 can be attributed to excess APTMS that could not be incorporated into the mesopores of SBA-15. A schematic representation of the APTMS incorporation based on the analysis results, is shown in Fig. 7.
Figure 8 shows SEM and TEM images of SBA-15, SBA(I)25, and SBA(HF)30. In the SEM images, SBA-15 exhibits rod-like particles with a diameter of approximately 1 μm, a morphology consistent with the findings of Zhao et al. [42]. The estimated wall thickness of pure SBA-15 ranged from 3 to 4 nm, which is also in agreement with the results reported by Zhao et al. [67].
Upon grafting APTMS onto SBA-15, the samples maintained their macrostructures and particle sizes, similar to pure SBA-15, which suggests that the morphology of SBA-15 remained intact during synthesis. The TEM images reveal that in all the samples, the channels were organized in parallel arrays, confirming that all the samples maintained ordered one-dimensional channels.
Figure 9 displays the spectra recorded by solid-state 13C dipolar decoupling (13C DD) MAS NMR and 29Si MAS NMR. In the 13C DD MAS NMR spectra of both SBA(I)25 and SBA(HF)30, signals appear at δ = 10.0, 26.3, and 43.6 ppm, attributed to the carbons within the propyl chain of grafted APTMS [68, 69].
The 29Si MAS NMR spectra of SBA-15 revealed resonances corresponding to Q2 ((SiO)2Si(OH)2), Q3 ((SiO)3SiOH), and Q4 ((SiO)4Si) sites at − 91.7, − 101.4, and − 110.4 ppm, respectively. Following APTMS modification, the signal at the Q2 site vanished. and the signal intensity of the Q3 site decreased. In the case of the SBA(I)25 and SBA(HF)30 samples, two new signals emerged at − 61.7 and − 68.7 ppm, attributed to the T2 ((SiO)2OHSiOR) and T3 ((SiO)3SiOR) sites, respectively.
To assess the APTMS state on the surface of SBA-15, we calculated the peak area ratios for the Q2, Q3, Q4, T2, and T3 sites, as summarized in Table 2. The results of the peak separation of 29Si NMR spectra are shown in Fig. S6. During synthesis, two types of condensation reactions occurred: one between APTMS and SBA-15 and the other among the APTMS molecules themselves. Thus, by examining the reduction in the number of surface silanol groups obtained from the Q2 and Q3 site peak areas and the increase in the T2 and T3 site peak areas, we calculated the degree of condensation between each component.
We calculated the ratio of the silyl groups of APTMS that reacted with the –SiOH groups on SBA-15 using Eq. (6), and we determined the utilization rate of the methoxy groups of the loaded APTMS molecules using Eqs. (7–9). Note that the sum of peak area ratios (Q2 + Q3 + Q4) is equal to 1.
where ΔQ2 and ΔQ3 are the reductions in the Q2 and Q3 site peak areas, respectively; and ΔT2 and ΔT3 are the increases in the T2 and T3 site peak areas, respectively. The methoxy group utilization rates are summarized in Table 3.
As indicated in Table 3, 21.3% of the methoxy groups from APTMS loaded onto SBA(I)25 reacted with the SiOH on SBA-15, which is more than twice that observed for SBA(HF)30 (9.7%). In contrast, 73.5% of the methoxy groups in SBA(HF)30 underwent intermolecular polymerization, indicating a 12.7% increase with respect to that of SBA(I)25. Consequently, the heating–filtration method exhibited a propensity to facilitate the condensation of APTMS molecules on the material surface, whereas the impregnation method tended to promote APTMS condensation on the surface of SBA-15.
To assess the effectiveness of silane utilization, we determined the actual amine loading on each adsorbent through a CHN analysis, and the nitrogen content of all samples were listed in Table S3. The amine immobilization efficiency is a crucial parameter that signifies the ratio of the actual amine loading to the quantity of amine introduced. As depicted in Fig. 10, the actual amine loadings of the SBA(I) samples increased with the increasing amine addition. Furthermore, the amine immobilization efficiencies of the SBA(I) samples consistently exceeded 90% for SBA(I)15, SBA(I)20, SBA(I)25, and SBA(I)30. This observation suggests that nearly all of the silane was successfully integrated into the solid state. In contrast, the efficiency of SBA(I)40 was 70.2%, possibly owing to the attachment of excess APTMS to the wall of the sample bottle, resulting in the loss of this reagent. Similarly, the actual amine loading of the SBA(HF) sample increased between 15 and 30 wt% (2 to 4.9 mmol g− 1), but when more amine was added, the actual amine loading remained constant. This behavior diverged from that of the SBA(I) samples, where excess APTMS was washed away by acetonitrile. When a small amount of amine was added, the SBA(HF) samples exhibited notably high amine immobilization efficiencies exceeding 91.9%. However, with increasing APTMS content, the amine immobilization efficiency decreased dramatically, reaching only 8.3% for SBA(HF)250. SBA(HF) samples with small amine additions (i.e., SBA(HF)15, SBA(HF)20, SBA(HF)25, SBA(HF)30, and SBA(HF)40) in this study had a similar loading amount of 3 mmol g–1, which is consistent with previous results [60, 62]. These findings indicate that excessive amine addition is unnecessary for preparing aminosilane-modified silica adsorbents.
The CO2 adsorption of the samples was measured at two CO2 concentrations, 400 ppm, and 5 vol%. Figure 11 illustrates the CO2-adsorption levels of the samples with various amine additions and actual loadings. Among the samples, SBA(I)25 and SBA(HF)30 exhibited the highest adsorption capacities. Specifically, under the 400 ppm CO2 flow, SBA(I)25 and SBA(HF)30 adsorbed 0.55 and 0.52 mmol g− 1 CO2, respectively, and under the 5 vol% CO2 flow, they adsorbed 1.0 and 1.1 mmol g− 1.
For the adsorbents, the nitrogen efficiency of adsorption under dry conditions was calculated using Eq. (10).
where nN is the number of moles of N in the adsorbent. Under the 400 ppm CO2 flow, the N efficiencies of SBA(I)25 and SBA(HF)30 were 40.4% and 36.3%, respectively, and under 5 vol% CO2, the corresponding efficiencies were 75.6% and 76.4%, respectively.
The amine addition in the heating–infiltration method was increased to 100 and 250 wt% to study the effects of excessive amine addition. With 400 ppm CO2, SBA(HF)100 and SBA(HF)250 adsorbed 0.48 and 0.33 mmol g–1, respectively, and under the 5 vol% CO2 flow, the CO2 adsorption amounts were 1.1 and 0.83 mmol g− 1. Thus, the amounts of CO2 adsorbed by SBA(HF)100 and SBA(HF)250 were similar to those by SBA(HF)30 and SBA(HF)20. This strongly indicates that excessive amine addition did not effectively improve the adsorption performance.
To understand the reason for this phenomenon, the relationship between the actual amine loading and adsorption amount was also plotted (Fig. 11, right). Evidently, the adsorption amount reached a maximum at actual amine loadings of 2.7 to 3 mmol g− 1 and then decreased when the actual amine loading exceeded 3 mmol g− 1. SBA(I)25 and SBA(HF)30 adsorbed the highest of amounts CO2, with actual amine loadings of 2.7 and 2.9 mmol g− 1, respectively. The actual amine loading amounts for SBA(HF)100 and SBA(HF)250 were 2.7 and 2.3 mmol g− 1, similar to those of SBA(HF)30 and SBA(HF)20, respectively. Additionally, the specific surface areas of SBA(HF)100 (151 m2 g− 1) and SBA(HF)250 (216 m2 g− 1) were comparable to those of SBA(HF)30 (139 m2 g− 1) and SBA(HF)20 (262 m2 g− 1), respectively. These results strongly indicate that the CO2 adsorption performance of APTMS-immobilized SBA-15 depends significantly on the actual amine loading amount rather than on other synthesis parameters, such as the amine addition and loading method.
Figure 12 illustrates how rapidly SBA(I) and SBA(HF) adsorbed CO2 under dry conditions. The adsorption rate of the SBA(I) samples was contingent upon the amine addition and actual amine loading. At 400 ppm, the adsorption rate of SBA(I) increased as the actual loading increased from 1 to 2 mmol g− 1 (corresponding to 15 and 20 wt% amine additions, respectively) but then slowed down as the actual loading increased from 2 to 4 mmol g− 1 (i.e., amine additions of 20–40 wt%). With 5 vol% CO2, the adsorption rate of SBA(I) increased with the actual loading increasing from 1 to 3 mmol g− 1 (15 to 25 wt% amine additions) and then decreased with the actual loading from 3 to 4 mmol g− 1 (25 to 40 wt% amine additions). The adsorption rates of the SBA(HF) samples under 5 vol% CO2 exhibited a similar trend to those of the SBA(I) samples, indicating that the adsorption rate was influenced by the number of adsorption sites and CO2 diffusion. Liu et al. [70] reported that excess amines become interconnected in the adsorbent, reducing the adsorption rate. Consequently, the decline in the adsorption rates at higher amine loadings can be attributed to the smaller pore size and pore blockage due to the high amine loading, which limits CO2 diffusion in the adsorbents.
In contrast, the adsorption rate trends for SBA(I) and SBA(HF) differed at 400 ppm. The SBA(HF) samples exhibited an adsorption rate of approximately 0.020 mmol g− 1min− 1 and did not change significantly with different amine additions, except for SBA(HF)100 and SBA(HF)250. Even when the actual amine loadings closely matched those of SBA(HF)30 and SBA(HF)20, the adsorption rates of SBA(HF)100 and SBA(HF)250 exceeded those of SBA(HF)30 and SBA(HF)20 by a considerable margin.
In the preceding section, the adsorbed amount was found to be strongly dependent on the amount of loaded amine. However, the adsorption rate, particularly at low CO2 concentrations, was influenced by other factors associated with amine loading. This can be attributed to alterations in the local structures and distribution of amine moieties. The 29Si NMR results (Fig. 9) reveal the varying oligomeric behaviors of the immobilized silanes in the samples synthesized using these two methods.
Tables 4 and 5 compare the performances of Class II adsorbents, along with the types of silane coupling agents, the weight ratios of silane added to the silica, the CO2 adsorption amounts, and the adsorption conditions. As presented in Tables 4 and 5, previous studies used more than twice as much aminosilane as silica by mass. Under dry CO2 conditions at 400 ppm (or close to 400 ppm), when compared to adsorbents that incorporated a high mono-aminosilane content (yielding CO2 adsorption amounts of 0.55 mmol g–1 [60] and 0.375 mmol g–1 [61]), our SBA(I)25 and SBA(HF)30 samples prepared with a lower silane utilization displayed comparable CO2 adsorption levels. Conversely, when compared to adsorbents with high di- or tri-aminosilane contents (resulting in CO2-adsorption amounts of 0.64 [62], 0.773 [63], 1.04 [71], 1.34 [72], and 0.98 [73] mmol g–1), SBA(I)25 and SBA(HF)30 exhibited lower adsorption capacities. This reduced adsorption can be attributed to the higher nitrogen content and increased number of adsorption sites in the di- and tri-aminosilanes. Under 5 vol% CO2, SBA(I)25 and SBA(HF)30 also demonstrated similar adsorption capacities (approximately 1.1 mmol g–1) to samples with high mono-aminosilane content. The adsorption amounts of SBA(I)25 and SBA(HF)30 at 50 °C under 5 vol% CO2 were also measured considering the high temperature of industrial exhaust gas conditions (Table S4). When the high temperature test was conducted, the adsorption amount of both adsorbents slightly decreased, which can be attributed to promoting the desorption of CO2 on adsorbents.
The efficiency of amine immobilization for the reported adsorbents was below 60%. In contrast, SBA(I)25 and SBA(HF)30 exhibited efficiencies of 92.0% and 78.3%, respectively, thus outperforming previously reported adsorbents. The other samples prepared with less than 30 wt% amine also displayed high immobilization efficiencies of approximately 90%. Conversely, samples with high amine contents, such as SBA(HF)100 and SBA(HF)250, demonstrated lower immobilization efficiencies, as detailed in Tables 4 and 5. The comparisons in Tables 4 and 5 strongly suggest that even with minimal usage of the silane coupling agent, the prepared adsorbents exhibited high adsorption performance, comparable to that of other Class II adsorption materials synthesized using more silane.
The results of CO2 adsorption/desorption stability measurements for SBA(I)25 and SBA(HF)30 under dry conditions are presented in Fig. 13. Under a dry CO2 flow of 400 ppm, the initial adsorption amounts on SBA(HF)30 and SBA(I)25 were 0.547 and 0.510 mmol g–1, respectively. Both samples exhibited negligible CO2 adsorption losses after three adsorption/desorption cycles. Furthermore, when subjected to the higher-concentration CO2 flow (5 vol%), the CO2 adsorption amounts for SBA(HF)30 and SBA(I)25 remained consistent at 1.092 and 1.025 mmol g–1, respectively, after three adsorption/desorption cycles. These results demonstrated that the synthesized adsorbents were highly stable.
CO2 adsorption kinetic behaviors of SBA(I)25 and SBA(HF)30 samples are also investigated. Three adsorption kinetic models, the Pseudo-first-order model [81], the Pseudo-second-order model [82], and the Avrami model [83] were used for fitting TG curves. The fitting results are shown in Fig S7, Fig S8 and Table S5. In the 400 ppm CO2, compared with the Pseudo-first-order model and Pseudo-second-order model, the degree of fitting based on the Avrami model is higher. As Chen et al. [84] pointed out in their report, CO2 adsorption over amine modified solid adsorbents is known to involve physical adsorption and chemical adsorption. Thus, the Avrami model, which incorporates both physical and chemical adsorption mechanisms, is well-fitted experimental data and is suited to describing the kinetics of CO2 adsorption over the amine-modified solid adsorbents. In contrast, in the 5 vol% CO2, there are no high degree of fitting on three models. This is probably due to the fast adsorption kinetics that is not suitable kinetic investigation and a change in the adsorption mechanism pointed out in the previous studies [84] and [85].
4 Conclusions
In this study, APTMS-modified SBA-15 adsorbents were successfully prepared with the aim of reducing the use of silane coupling agents. The adsorbents were synthesized through heating–filtration and impregnation processes, and acetonitrile was identified as the optimal solvent for both reactions. The grafting of APTMS onto SBA-15 was investigated through N2 adsorption characterization, XRD, NMR, TEM, and elemental analysis. Notably, in both synthesis methods, the aminosilane preferentially blocked the micropores of SBA-15. In the impregnation method, as the amine loading increased, it gradually occupied the mesopores until they were completely blocked. However, in the heating–filtration method, even under excessive amine addition conditions, the prepared samples retained their mesopore structure.
Under dry conditions with a 400 ppm CO2 gas flow at 30 °C, both SBA(I)25 and SBA(HF)30 exhibited high adsorption levels of approximately 0.5 mmol g–1. Similarly, using dry 5 vol% CO2 gas flow at 30 °C, both SBA(I)25 and SBA(HF)30 adsorbed approximately 1.0 mmol g–1. Although the amounts adsorbed by the SBA(I) and SBA(HF) samples were comparable, these two adsorbents exhibited different adsorption rates, particularly under low-concentration CO2. Moreover, both samples demonstrated excellent recycling stability under dry conditions. According to the results of the adsorption kinetic study, the Avrami model can simulate well with the experimental data, indicating that the major CO2 adsorption of SBA(I)25 and SBA(HF)30 is chemical adsorption.
Compared to previously reported adsorbents, SBA(I)25 and SBA(HF)30 exhibited similar adsorption capacities to those of conventional Class II materials. However, the amine immobilization efficiency of the present materials was notably high at approximately 90%. As noted by Hu et al. [86], the study of N-functionalized solid adsorbents is still in the stages of selecting materials and determining the optimal adsorption conditions. Considering the recent rapid development of CCUS technology, the cost of CO2 adsorbents has become increasingly significant for practical applications. Consequently, this discovery holds promise for the economic development and large-scale production of Class II adsorbents.
Data availability
No datasets were generated or analysed during the current study.
References
L.Y. Liu, H.G. Ji, X.F. Lü, T. Wang, S. Zhi, F. Pei, D.L. Quan, Int. J. Min. Metall. Mater. 28, 513 (2021). https://doi.org/10.1007/s12613-020-2155-4
O.E. Aksyutin, A.G. Ishkov, K.V. Romanov, V.A. Grachev, Int. J. GEOMATE. 16, 153 (2019)
L. Al-Ghussain, Environ. Prog Sustain. Energy. 38, 13 (2019). https://doi.org/10.1002/ep.13041
Z. Hu, J.W. Lee, K. Chandran, S. Kim, S.K. Khanal, Environ. Sci. Technol. 46, 6470 (2012). https://doi.org/10.1021/es300110x
H. Tian, R. Xu, J.G. Canadell, R.L. Thompson, W. Winiwarter, P. Suntharalingam, E.A. Davidson, P. Ciais, R.B. Jackson, G. Janssens-Maenhout, M.J. Prather, P. Regnier, N. Pan, S. Pan, G.P. Peters, H. Shi, F.N. Tubiello, S. Zaehle, F. Zhou, A. Arneth, G. Battaglia, S. Berthet, L. Bopp, A.F. Bouwman, E.T. Buitenhuis, J. Chang, M.P. Chipperfield, S.R.S. Dangal, E. Dlugokencky, J.W. Elkins, B.D. Eyre, B. Fu, B. Hall, A. Ito, F. Joos, P.B. Krummel, A. Landolfi, G.G. Laruelle, R. Lauerwald, W. Li, S. Lienert, T. Maavara, M. MacLeod, D.B. Millet, S. Olin, P.K. Patra, R.G. Prinn, P.A. Raymond, D.J. Ruiz, G.R. van der Werf, N. Vuichard, J. Wang, R.F. Weiss, K.C. Wells, C. Wilson, J. Yang, Y. Yao, Nature. 586, 248 (2020). https://doi.org/10.1038/s41586-020-2780-0
V. Masson-Delmotte, P. Zhai, H.-O. Pörtner, D. Roberts, J. Skea, P.R. Shukla, A. Pirani, W. Moufouma-Okia, C. Péan, R. Pidcock, S. Connors, J.B.R. Matthews, Y. Chen, X. Zhou, M.I. Gomis, E. Lonnoy, T. Maycock, M. Tignor, T. Waterfield, Global Warming of 1.5°C, IPCC, (2018)
M. Bui, C.S. Adjiman, A. Bardow, E.J. Anthony, A. Boston, S. Brown, P.S. Fennell, S. Fuss, A. Galindo, L.A. Hackett, J.P. Hallett, H.J. Herzog, G. Jackson, J. Kemper, S. Krevor, G.C. Maitland, M. Matuszewski, I.S. Metcalfe, C. Petit, G. Puxty, J. Reimer, D.M. Reiner, E.S. Rubin, S.A. Scott, N. Shah, B. Smit, J.P.M. Trusler, P. Webley, J. Wilcox, N. Mac Dowell, Energy Environ. Sci. 11, 1062 (2018). https://doi.org/10.1039/C7EE02342A
L. Zhang, Y. Song, J. Shi, Q. Shen, D. Hu, Q. Gao, W. Chen, K.-W. Kow, C. Pang, N. Sun, W. Wei, Adv. Atmos. Sci. 39, 1252 (2022). https://doi.org/10.1007/s00376-022-1467-x
S. Lian, C. Song, Q. Liu, E. Duan, H. Ren, Y. Kitamura, J. Environ. Sci. 99, 281 (2021). https://doi.org/10.1016/j.jes.2020.06.034
R.S. Haszeldine, Science. 325, 1647 (2009). https://doi.org/10.1126/science.1172246
G. Bai, Y. Han, P. Du, Z. Fei, X. Chen, Z. Zhang, J. Tang, M. Cui, Q. Liu, X. Qiao, New. J. Chem. 43, 18345 (2019). https://doi.org/10.1039/C9NJ03822A
H.M. Stowe, G.S. Hwang, Ind. Eng. Chem. Res. 56, 6887 (2017). https://doi.org/10.1021/acs.iecr.7b00213
I.M. Saeed, P. Alaba, S.A. Mazari, W.J. Basirun, V.S. Lee, N. Sabzoi, Int. J. Greenh. Gas Control. 79, 212 (2018). https://doi.org/10.1016/j.ijggc.2018.11.002
N. Kladkaew, R. Idem, P. Tontiwachwuthikul, C. Saiwan, Energy Procedia. 4, 1761 (2011). https://doi.org/10.1016/j.egypro.2011.02.051
L.M. Romeo, I. Bolea, J.M. Escosa, Appl. Therm. Eng. 28, 1039 (2008). https://doi.org/10.1016/j.applthermaleng.2007.06.036
Z. Liang, K. Fu, R. Idem, P. Tontiwachwuthikul, Chin. J. Chem. Eng. 24, 278 (2016). https://doi.org/10.1016/j.cjche.2015.06.013
P. Zhao, G. Zhang, H. Yan, Y. Zhao, Chin. J. Chem. Eng. 35, 17 (2021). https://doi.org/10.1016/j.cjche.2020.11.028
L.A. Darunte, K.S. Walton, D.S. Sholl, C.W. Jones, Curr. Opin. Chem. Eng. 12, 82 (2016). https://doi.org/10.1016/j.coche.2016.03.002
A.A. Azmi, M.A.A. Aziz, J. Environ. Chem. Eng. 7, 103022 (2019). https://doi.org/10.1016/j.jece.2019.103022
L. Lin, Y. Meng, T. Ju, S. Han, F. Meng, J. Li, Y. Du, M. Song, T. Lan, J. Jiang, J. Environ. Manage. 325/A, 116438 (2023). https://doi.org/10.1016/j.jenvman.2022.116438
D. Panda, V. Kulkarni, S.K. Singh, React. Chem. Eng. 8, 10 (2022). https://doi.org/10.1039/D2RE00211F
C.N. Okonkwo, C. Okolie, A. Sujan, G. Zhu, C.W. Jones, Energy Fuels. 32, 6926 (2018). https://doi.org/10.1021/acs.energyfuels.8b00936
A. Ayub, S. Ahsan, D. Meeroff, M.J. Lashaki, Chem. Eng. J. 445, 136497 (2022). https://doi.org/10.1016/j.cej.2022.136497
T. Gelles, S. Lawson, A.A. Rownaghi, F. Rezaei, Adsorption. 26, 5 (2020). https://doi.org/10.1007/s10450-019-00151-0
N.N. Linneen, R. Pfeffer, Y.S. Lin, Chem. Eng. J. 254, 190 (2014). https://doi.org/10.1016/j.cej.2014.05.087
J.-T. Anyanwu, Y. Wang, R.T. Yang, Chem. Eng. Sci. 254, 117626 (2022). https://doi.org/10.1016/j.ces.2022.117626
X. Wang, L. Chen, Q. Guo, Chem. Eng. J. 260, 573 (2015). https://doi.org/10.1016/j.cej.2014.08.107
M.J. Lashaki, S. Khiavi, A. Sayari, Chem. Soc. Rev. 48, 3320 (2019). https://doi.org/10.1039/c8cs00877a
C.-H. Yu, C.-H. Huang, C.-S. Tan, Aerosol Air Qual. Res. 12, 745 (2012). https://doi.org/10.4209/aaqr.2012.05.0132
C. Chen, S. Zhang, K.H. Row, W.-S. Ahn, J. Energy Chem. 26, 868 (2017). https://doi.org/10.1016/j.jechem.2017.07.001
C. Chen, J. Kim, W.-S. Ahn, Korean J. Chem. Eng. 31, 1919 (2014). https://doi.org/10.1007/s11814-014-0257-2
L. Nie, Y. Mu, J. Jin, J. Chen, J. Mi, Chin. J. Chem. Eng. 26, 2303 (2018). https://doi.org/10.1016/j.cjche.2018.07.012
A. Samanta, A. Zhao, G.K.H. Shimizu, P. Sarkar, R. Gupta, Ind. Eng. Chem. Res. 51, 1438 (2012). https://doi.org/10.1021/ie200686q
V. Zeleňák, M. Badaničová, D. Halamová, J. Čejka, A. Zukal, N. Murafa, G. Goerigk, Chem. Eng. J. 144, 336 (2008). https://doi.org/10.1016/j.cej.2008.07.025
F.-Y. Chang, K.-J. Chao, H.-H. Cheng, C.-S. Tan, Sep. Purif. Technol. 70, 87 (2009). https://doi.org/10.1016/j.seppur.2009.08.016
H.A. Patel, J. Byun, C.T. Yavuz, ChemSusChem 10, 1303 (2017). https://doi.org/10.1002/cssc.201601545
M.S. Yilmaz, J. Therm, Anal. Calorim. 146, 2241 (2021). https://doi.org/10.1007/s10973-020-10417-3
Y.G. Ko, H.J. Lee, H.C. Oh, U.S. Choi, J. Hazard. Mater. 250–251 (2013). https://doi.org/10.1016/j.jhazmat.2013.01.035
R. Kishor, A.K. Ghoshal, Micropor Mesopor Mater. 246, 137 (2017). https://doi.org/10.1016/j.micromeso.2017.03.023
J. de Ribeiro, E.H.M. Nunes, D.C.L. Vasconcelos, W.L. Vasconcelos, J.F. Nascimento, W.M. Grava, P.W.J. Derks, J. Porous Mater. 26, 1581 (2019). https://doi.org/10.1007/s10934-019-00754-6
N. Minju, B.N. Nair, A.P. Mohamed, S. Ananthakumar, Sep. Purif. Technol. 181, 192 (2017). https://doi.org/10.1016/j.seppur.2017.03.038
D. Zhao, J. Feng, Q. Huo, N. Melosh, G.H. Fredrickson, B.F. Chmelka, G.D. Stucky, Science. 279, 548 (1998). https://doi.org/10.1126/science.279.5350.548
C.-M. Yang, B. Zibrowius, W. Schmidt, F. Schüth, Chem. Mater. 16, 2918 (2004). https://doi.org/10.1021/cm049526z
M. Erans, E.S. Sanz-Pérez, D.P. Hanak, Z. Clulow, D.M. Reiner, G.A. Mutch, Energy Environ. Sci. 15, 1360 (2022). https://doi.org/10.1039/D1EE03523A
L. Zhang, Y. Zhao, H. Dai, H. He, C.T. Au, Catal. Today. 131, 42 (2008). https://doi.org/10.1016/j.cattod.2007.10.017
G. Jakša, B. Štefane, J. Kovač, Appl. Surf. Sci. 315, 516 (2014). https://doi.org/10.1016/j.apsusc.2014.05.157
T. Song, P. Zhang, C. Zhang, L. Gong, X. Cao, B. Wang, R. Yu, W. Zhou, Micropor Mesopor Mater. 334, 111761 (2022). https://doi.org/10.1016/j.micromeso.2022.111761
M.J. Lashaki, A. Sayari, Chem. Eng. J. 334, 1260 (2018). https://doi.org/10.1016/j.cej.2017.10.103
M. Gil, I. Tiscornia, Ó. de la Iglesia, R. Mallada, J. Santamaría, Chem. Eng. J. 175, 291 (2011). https://doi.org/10.1016/j.cej.2011.09.107
Y. Li, N. Sun, L. Li, N. Zhao, F. Xiao, W. Wei, Y. Sun, W. Huang, Mater. (Basel). 6, 981 (2013). https://doi.org/10.3390/ma6030981
G. Zhang, P. Zhao, L. Hao, Y. Xu, J. CO2 Util. 24, 22 (2018). https://doi.org/10.1016/j.jcou.2017.12.006
R. Kishor, A.K. Ghoshal, Chem. Eng. J. 262, 882 (2015). https://doi.org/10.1016/j.cej.2014.10.039
X. Wang, K.S. Lin, J.C. Chan, S. Cheng, J. Phys. Chem. B 109, 1763 (2005). https://doi.org/10.1021/jp045798d
P. Shah, N. Sridevi, A. Prabhune, V. Ramaswamy, Micropor Mesopor Mater. 116, 157 (2008). https://doi.org/10.1016/j.micromeso.2008.03.030
M. Laghaei, M. Sadeghi, B. Ghalei, M. Dinari, Prog Org. Coat. 90, 163 (2016). https://doi.org/10.1016/j.porgcoat.2015.10.007
S. Hao, H. Chang, Q. Xiao, Y. Zhong, W. Zhu, J. Phys. Chem. C 115, 12873 (2011). https://doi.org/10.1021/jp200252u
T. Yarbaş, A. Çıtak, I.J. Adhes, Adhes. 118, 103249 (2022). https://doi.org/10.1016/j.ijadhadh.2022.103249
Z. El Berrichi, B. Louis, J.P. Tessonnier, O. Ersen, L. Cherif, M.J. Ledoux, C. Pham-Huu, Appl. Catal. A 316, 219 (2007). https://doi.org/10.1016/j.apcata.2006.09.033
I. Eswaramoorthi, A.K. Dalai, Micropor Mesopor Mater. 93, 1 (2006). https://doi.org/10.1016/j.micromeso.2006.01.018
Y. Wang, J.-T. Anyanwu, Z. Hu, R.T. Yang, Sep. Purif. Technol. 309, 123030 (2023). https://doi.org/10.1016/j.seppur.2022.123030
Y. Wang, R.T. Yang, A.C.S. Sustainable Chem, Eng. 8, 8295 (2020). https://doi.org/10.1021/acssuschemeng.0c01941
E.S. Sanz-Pérez, A. Fernández, A. Arencibia, G. Calleja, R. Sanz, Chem. Eng. J. 373, 1286 (2019). https://doi.org/10.1016/j.cej.2019.05.117
J.-T. Anyanwu, Y. Wang, R.T. Yang, Ind. Eng. Chem. Res. 59, 7072 (2020). https://doi.org/10.1021/acs.iecr.9b05228
E. Da’na, Micropor Mesopor Mater. 247, 145 (2017). https://doi.org/10.1016/j.micromeso.2017.03.050
R.A. Pollock, B.R. Walsh, J. Fry, I.T. Ghampson, Y.B. Melnichenko, H. Kaiser, R. Pynn, W.J. DeSisto, M.C. Wheeler, B.G. Frederick, Chem. Mater. 23, 3828 (2011). https://doi.org/10.1021/cm200707y
Y. Millot, A. Hervier, J. Ayari, N. Hmili, J. Blanchard, S. Boujday, J. Am. Chem. Soc. 145, 6671 (2023). https://doi.org/10.1021/jacs.2c11390
A. Zhao, A. Samanta, P. Sarkar, R. Gupta, Ind. Eng. Chem. Res. 52, 6480 (2013). https://doi.org/10.1021/ie3030533
A.M. Chong, X. Zhao, J. Phys. Chem. B 107, 12650 (2003)
P.D. Young, J.M. Notestein, ChemSusChem. 4, 1671 (2011). https://doi.org/10.1002/cssc.201100244
L. Liu, S. Jin, K. Ko, H. Kim, I.-S. Ahn, C.-H. Lee, Chem. Eng. J. 382, 122834 (2020). https://doi.org/10.1016/j.cej.2019.122834
J.-T. Anyanwu, Y. Wang, R.T. Yang, Chem. Eng. J. 427, 131561 (2022). https://doi.org/10.1016/j.cej.2021.131561
Y. Belmabkhout, R. Serna-Guerrero, A. Sayari, Ind. Eng. Chem. Res. 49, 359 (2010). https://doi.org/10.1021/ie900837t
C.-J. Yoo, L.-C. Lee, C.W. Jones, Langmuir. 31, 13350 (2015). https://doi.org/10.1021/acs.langmuir.5b03657
F. Bai, X. Liu, Y. Liu, M. Li, S. Sani, W. Guo, C. Sun, Micropor Mesopor Mater. 349, 112370 (2023). https://doi.org/10.1016/j.micromeso.2022.112370
V. Zelenak, D. Halamova, L. Gaberova, E. Bloch, P. Llewellyn, Micropor Mesopor Mater. 116, 358 (2008). https://doi.org/10.1016/j.micromeso.2008.04.023
J. Wei, J. Shi, H. Pan, W. Zhao, Q. Ye, Y. Shi, Micropor Mesopor Mater. 116, 394 (2008). https://doi.org/10.1016/j.micromeso.2008.04.028
M. Yao, Y. Dong, X. Feng, X. Hu, A. Jia, G. Xie, G. Hu, J. Lu, M. Luo, M. Fan, Fuel. 123, 66 (2014). https://doi.org/10.1016/j.fuel.2014.01.059
L. Wang, L. Ma, A. Wang, Q. Liu, T. Zhang, Chin. J. Catal. 28, 805 (2007). https://doi.org/10.1016/S1872-2067(07)60066-7
H. Nigar, B. Garcia-Baños, F.L. Peñaranda‐Foix, J.M. Catalá‐Civera, R. Mallada, J. Santamaría, AIChE J. 62, 547 (2016). https://doi.org/10.1002/aic.15118
F. Bai, X. Liu, S. Sani, Y. Liu, W. Guo, C. Sun, Sep. Purif. Technol. 299, 121539 (2022). https://doi.org/10.1016/j.seppur.2022.121539
F. Liu, Y. Lou, F. Xia, B. Hu, Chem. Eng. J. 454, 140318 (2023). https://doi.org/10.1016/j.cej.2022.140318
F. Liu, Y. Lou, F. Xia, B. Hu, Chem. Eng. J. 456, 141100 (2023). https://doi.org/10.1016/j.cej.2022.141100
Z. Gu, X. Wang, P. Huang, Y. Huang, X. He, X. Wei, J. Yue, J. Jiang, C. Zhao, Process. Saf. Environ. 160, 573 (2022). https://doi.org/10.1016/j.psep.2022.02.047
S. Chen, B. Jia, Y. Peng, X. Luo, Y. Huang, B. Jin, H. Gao, Z. Liang, X. Hu, Y. Zhou, Ind. Eng. Chem. Res. 60, 17150 (2021). https://doi.org/10.1021/acs.iecr.1c03436
R. Serna-Guerrero, A. Sayari, Chem. Eng. J. 161, 182 (2010). https://doi.org/10.1021/ie801186g
X.E. Hu, L. Liu, X. Luo, G. Xiao, E. Shiko, R. Zhang, X. Fan, Y. Zhou, Y. Liu, Z. Zeng, C. Li, Appl. Energy. 260, 114244 (2020). https://doi.org/10.1016/j.apenergy.2019.114244
Acknowledgements
This study was funded by the International Network on Polyoxometalate Science at Hiroshima University, the New Energy and Industrial Technology Development Organization (NEDO), through project JPNP20004 and the Feasibility Study Program (Uncharted Territory Challenge 2050) JPNP14004, the JST SICORP program through Grant Number JPMJSC22C5, JSPS KAKENHI Grant Number 22H01868, a Grant-in-Aid for Transformative Research Area (A) “Supra-ceramics” (JSPS KAKENHI Grant Number JP22H05144), and the JSPS Core-to-Core Program.
Funding
Open Access funding provided by Hiroshima University.
Author information
Authors and Affiliations
Contributions
Jinrui Li: Materials synthesis and characterization, original draft preparation, reviewing, and editing; Nao Tsunoji: Conceptualization and methodology, original draft preparation, funding acquisition, reviewing, and editing; Rajesh Kumar: Materials characterization; Ndaru Candra Sukmana: Materials characterization; Masahiro Sadakane: Materials synthesis, reviewing, and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, J., Tsunoji, N., Kumar, R. et al. Minimizing usage of silane coupling agent for amine-grafted mesoporous silica CO2 adsorbent. J Porous Mater 31, 1289–1304 (2024). https://doi.org/10.1007/s10934-024-01596-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-024-01596-7