Abstract
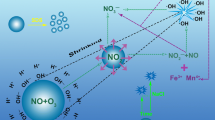
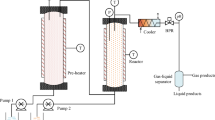
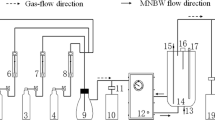
As an eco-friendly technology, micro-nano bubbles have gained extensive attention due to their excellent properties. We carried out the experiments to investigate the degradation performance of micro-nano bubbles on ethyl acetate at ambient temperature and pressure. The effects were deeply analyzed by studying the treatment time, initial concentration, and mixed components on ethyl acetate. Treatment time at 30 min had the best results, with a removal efficiency of 86.07 % and a degradation rate of 0.340 ± 0.021 min−1. With the increase of the initial ethyl acetate concentration, the degradation extent first increased and then decreased. The best efficiency of 94.61% and the maximum reaction rate of 8.79×10−3 min−1 were achieved at an initial concentration of 265.6 mg/m3. In addition, ethyl acetate degradation was inhibited by the presence of butyl acetate, and removal efficiency of mixed components was lower than that of single components. The GC-MS results showed that possible intermediates, such as ethanol and acetone, were produced during the decomposition process, which was expected to eventually decompose into CO2 and H2O as the reaction progresses. This work presents a new method for the degradation of ethyl acetate and provides valuable information for the degradation of organic matter by micro-nano bubbles.
Graphical abstract







Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this article and its supplementary material.
References
Andreozzi R, Caprio V, Insola A, Marotta R (1999) Advanced oxidation processes (AOP) for water purification and recovery. Catal today 53:51–59. https://doi.org/10.1016/S0920-5861(99)00102-9
Barbusinski K, Kalemba K, Kasperczyk D, Urbaniec K, Kozik V (2017) Biological methods for odor treatment: a review. J Cleaner Prod 152:223–241. https://doi.org/10.1016/j.jclepro.2017.03.093
Cai YX, Zhu XB, Hu WS, Zheng CH, Yang Y, Chen MH, Gao X (2019) Plasma-catalytic decomposition of ethyl acetate over LaMO3 (M=Mn, Fe, and Co) perovskite catalysts. J Ind Eng Chem 70:447–452. https://doi.org/10.1016/j.jiec.2018.11.007
Cui JH, Liu SJ, Xue H, Wang XQ, Hao ZQ, Liu R, Shang W, Zhao D et al (2021) Catalytic ozonation of volatile organic compounds (ethyl acetate) at normal temperature. Chin J Chem Eng 32:159–167. https://doi.org/10.1016/j.cjche.2020.09.021
Guo T, Cheng G, Tan G, Xu L, Huang Z, Cheng P, Zhou Z (2021) Real-time analysis of intermediate products from non-thermal plasma degradation of ethyl acetate in air using PTR-MS: performance evaluation and mechanism study. Chemosphere 264:128430. https://doi.org/10.1016/j.chemosphere.2020.128430
Haque R, Saxena M, Shit SC, Asokan P (2015) Fibre-matrix adhesion and properties evaluation of sisal polymer composite. Fibers Polym 16:146–152. https://doi.org/10.1007/s12221-015-0146-2
Jiang Y, Pétrier C, Waite TD (2002) Kinetics and mechanisms of ultrasonic degradation of volatile chlorinated aromatics in aqueous solutions. Ultrason Sonochem 9:317–323. https://doi.org/10.1016/S1350-4177(02)00085-8
Jiang YW, Gao JH, Zhang Q, Liu ZY, Fu ML, Wu JL, Hu Y, Ye DQ (2019) Enhanced oxygen vacancies to improve ethyl acetate oxidation over MnOx-CeO2 catalyst derived from MOF template. Chem Eng J 371:78–87. https://doi.org/10.1016/j.cej.2019.03.233
Khan P, Zhu WJ, Huang F, Gao WL, Khan NA (2020) Micro-nanobubble technology and water-related application. Water Supply 20:2021–2035. https://doi.org/10.2166/ws.2020.121
Lin CW, Tsao CY, Jiang SL, Liu SH (2018) Enhanced gaseous ethyl acetate degradation and power generation by a bioelectrochemical system. Chem Eng J 344:270–276. https://doi.org/10.1016/j.cej.2018.03.093
Litter MI (2005) Introduction to photochemical advanced oxidation processes for water treatment. In: Boule P, Bahnemann DW, Robertson PKJ (eds) The Handbook of Environmental Chemistry, vol 2M. Springer, Berlin, pp 325–366. https://doi.org/10.1007/b138188
Liu JG, Diamond J (2008) Revolutionizing China’s environmental protection. Science 319:37–38. https://doi.org/10.1126/science.1150416
Liu X, Liu JQ, Chen JY, Zhong FC (2021) Mn2O3/gamma-Al2O3 catalysts synergistic double dielectric barrier discharge (DDBD) degradation of toluene, ethyl-acetate and acetone. Chemosphere 284:131299. https://doi.org/10.1016/j.chemosphere.2021.131299
Lovascio S, Blin-Simiand N, Magne L, Jorand F, Pasquiers S (2014) Experimental study and kinetic modeling for ethanol treatment by air dielectric barrier discharges. Plasma Chem and Plasma Process 35:279–301. https://doi.org/10.1007/s11090-014-9601-x
Ma M, Yang R, Jiang ZY, Chen CW, Liu QY, Albilali R, He C (2021a) Fabricating M/Al2O3/cordierite (M=Cr, Mn, Fe, Co, Ni and Cu) monolithic catalysts for ethyl acetate efficient oxidation: unveiling the role of water vapor and reaction mechanism. Fuel 303:121244. https://doi.org/10.1016/j.fuel.2021.121244
Ma M, Yang R, He C, Jiang ZY, Shi JW, Albilali R, Fayaz K, Liu B (2021b) Pd-based catalysts promoted by hierarchical porous Al2O3 and ZnO microsphere supports/coatings for ethyl acetate highly active and stable destruction. J Hazard Mater 401:123281. https://doi.org/10.1016/j.jhazmat.2020.123281
Mondal K, Rajakumar B (2020) Kinetic investigations on the reaction of phenyl radicals with ethyl acetate in the gas phase: an experimental and computational study. J Phys Chem A 124:5503–5512. https://doi.org/10.1021/acs.jpca.0c03872
Mosley S (2014) Environmental history of air pollution and protection. In: Agnoletti M, Neri-Serneri S (eds) The basic environmental history. Springer, Cham, pp 143–169. https://doi.org/10.1007/978-3-319-09180-8_5
Mudliar S, Giri B, Padoley K, Satpute D, Dixit R, Bhatt P, Pandey R, Juwarkar A et al (2010) Bioreactors for treatment of VOCs and odours: a review. J Environ Manage 91:1039–1054. https://doi.org/10.1016/j.jenvman.2010.01.006
Natarajan R (2020) Performance evaluation of biodegradation of isoprene-acetone mixture in integrated biofilter. J Environ Eng 146:4020030. https://doi.org/10.1061/(ASCE)EE1943-7870.0001711
Natarajan R, Al-Sinani J, Viswanathan S, Manivasagan R (2017) Biodegradation of ethyl benzene and xylene contaminated air in an up flow mixed culture biofilter. Int Biodeterior Biodegrad 119:309–315. https://doi.org/10.1016/j.ibiod.2016.10.041
Nguyen VT, Nguyen DB, Mok YS, Hossain MM, Saud S, Yoon KH, Dinh DK, Ryu S et al (2021) Removal of ethyl acetate in air by using different types of corona discharges generated in a honeycomb monolith structure coated with Pd/gamma-alumina. J Hazard Mater 416:126162. https://doi.org/10.1016/j.jhazmat.2021.126162
Pee GY, Rathman JF, Weavers LK (2004) Effects of surface active properties on the cavitational degradation of surfactant contaminants. Ind Eng Chem Res 43:5049–5056. https://doi.org/10.1021/ie0306022
Perillo R, Ferracin E, Giardina A, Marotta E, Paradisi C (2019) Efficiency, products and mechanisms of ethyl acetate oxidative degradation in air non-thermal plasma. J Phys D: Appl Phys 52:295206. https://doi.org/10.1088/1361-6463/ab1aff
Saharan VK, Badve MP, Pandit AB (2011) Degradation of reactive red 120 dye using hydrodynamic cavitation. Chem Eng J 178:100–107. https://doi.org/10.1016/j.cej.2011.10.018
Saravanan V, Rajasimman M, Rajamohan N (2013) Biofiltration of volatile organic compound using two packing materials: kinetics and modelling. Korean J Chem Eng 30:1918–1928. https://doi.org/10.1007/s11814-013-0113-9
Saravanan V, Rajasimman M, Rajamohan N (2014) Performance of packed bed biofilter during transient operating conditions on removal of xylene vapour. Int J Environ Sci Technol 12:1625–1634. https://doi.org/10.1007/s13762-014-0521-3
Singh RL, Singh PK (2017) Global Environmental Problems. In: Singh R (ed) Principles and applications of environmental biotechnology for a sustainable future. Springer, Singapore, pp 13–41. https://doi.org/10.1007/978-981-10-1866-4_2
Tada K, Maeda M, Nishiuchi Y, Nagahara J, Hata T, Zhao ZW, Yoshida Y, Watanabe S et al (2014) ESR measurement of hydroxyl radicals in micro-nanobubble water. Chem Lett 43:1907–1908. https://doi.org/10.1246/cl.140691
Takahashi M (2010) Wastewater treatment technology using microbubbles. Nihonkaisui Science Journal 64:19–23. https://doi.org/10.11457/swsj.64.19
Viswanathan S, Rajasimman M, Natarajan R (2012) Experimental determination of kinetic parameters for the removal of ethylacetate and xylene in a biofilter. Int J Chem React Eng 10:A76. https://doi.org/10.1515/1542-6580.3015
Wang HQ, Chen S, Wang Z, Zhou Y, Wu ZB (2019) A novel hybrid Bi2MoO6-MnO2 catalysts with the superior plasma induced pseudo photocatalytic-catalytic performance for ethyl acetate degradation. Appl Catal B 254:339–350. https://doi.org/10.1016/j.apcatb.2019.05.018
Wu CH, Lin CW (2016) Electricity generation and kinetic aspects of a biotrickling filter-microbial fuel cell for the biofiltration of ethyl acetate vapor from waste gas. J Taiwan Inst Chem Eng 68:332–337. https://doi.org/10.1016/j.jtice.2016.09.023
Wu CH, Shih JC, Lin CW (2016) Continuous production of power using microbial fuel cells with integrated biotrickling filter for ethyl acetate-contaminated air stream treatment. Int J Hydrogen Energy 41:21945–21954. https://doi.org/10.1016/j.ijhydene.2016.08.186
Xiao ZG, Aftab TB, Li DX (2019) Applications of micro-nano bubble technology in environmental pollution control. Micro Nano Lett 14:782–787. https://doi.org/10.1049/mnl.2018.5710
Yang Y, Li YZ, Zhang Q, Zeng M, Wu SW, Lan L, Zhao XJ (2018) Novel photoactivation and solar-light-driven thermocatalysis on ε-MnO2 nanosheets lead to highly efficient catalytic abatement of ethyl acetate without acetaldehyde as unfavorable by-product. J Mater Chem A 6:14195–14206. https://doi.org/10.1039/C8TA04274H
Ye YF, Zhu Y, Lu N, Wang X, Su Z (2021) Treatment of rhodamine B with cavitation technology: comparison of hydrodynamic cavitation with ultrasonic cavitation. RSC Adv 11:5096–5106. https://doi.org/10.1039/D0RA07727E
Zhang XY, Gao B, Creamer AE, Cao CC, Li YC (2017) Adsorption of VOCs onto engineered carbon materials: a review. J Hazard Mater 338:102–123. https://doi.org/10.1016/j.jhazmat.2017.05.013
Zhao YF, Gao J, Cai YJ, Wang JJ, Pan J (2021) Real-time tracing VOCs, O3 and PM2.5 emission sources with vehicle-mounted proton transfer reaction mass spectrometry combined differential absorption lidar. Atmos Pollut Res 12:146–153. https://doi.org/10.1016/j.apr.2021.01.008
Author information
Authors and Affiliations
Contributions
Juan Hu, Ya-zhuo Hao, and Jian-jun Wei provided ideas for the experiment. The elementary experiments were operated by Juan Hu and Ya-zhuo Hao. Zhong-ming Guo and William Bai provided maintenance and material support for the experiment. Jian-jun Wei reviewed and edited the manuscript. Juan Hu wrote the original manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Hu, J., Hao, Yz., Wei, Jj. et al. Influencing factors and kinetics study on the degradation of gaseous ethyl acetate by micro-nano bubbles. Environ Sci Pollut Res 29, 77275–77282 (2022). https://doi.org/10.1007/s11356-022-21063-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-21063-7