Abstract
Rate constants of chlorine atom (Cl•) reactions (kCl•) determined using a large variation of experimental methods, including transient measurements, steady-state and computation techniques, were collected from the literature and were discussed together with the reaction mechanisms. The kCl• values are generally in the 108–109 mol−1 dm3 s−1 range when the basic reaction between the Cl• and the target molecule is H-atom abstraction. When Cl• addition to double bonds dominates the interaction, the kCl• values are in the 1 × 109–2 × 1010 mol−1 dm3 s−1 range. In the kCl• = 1 × 1010–4 × 1010 mol−1 dm3 s−1 range, single-electron-transfer reactions may also contribute to the mechanism. The Cl• reactions with organic molecules in many respects are similar to those of •OH, albeit Cl• seems to be less selective as •OH. However, there is an important difference, as opposed to Cl• in the case of •OH single-electron-transfer reactions have minor importance. The uncertainty of Cl• rate constant determinations is much higher than those of •OH. Since Cl• reactions play very important role in the emerging UV/chlorine water purification technology, some standardization of the rate constant measuring techniques and more kCl• measurements are recommended.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently the UV/chlorine technique has been investigated as an alternative to the UV/H2O2 advanced oxidation process (AOP) and has been tested at a few water treatment utilities (Jin et al. 2011; De Laat and Stefan 2018; Zhang et al. 2018; Kishomoto 2019). The degradation of organic pollutants during the UV/chlorine process takes place by several parallel reactions, e.g., direct photolysis, if the organic compounds absorb the UV radiation used, oxidation by the free radicals (hydroxyl radical (•OH), chlorine atom (Cl•) and also other radical species). In aqueous solutions, the free chlorine species exist in three forms: at low pH the Cl2 form, between pH 1 and 8 the HOCl form and at high pH ClO– form dominates.
The UV photolysis of HOCl gives •OH and Cl• with quantum yields close to unity. With lower yield, Cl• also forms in the photolysis of ClO–.
From these equations, it is obvious that the reactions of the chlorine atom play an important role during the UV/chlorine-advanced oxidation process. In such systems, •OH is always present. Based on reactions (3)–(4), equal yields of Cl• and •OH are expected. However, in practice the •OH yield is higher at high HOCl concentrations, since Cl• may abstract an H-atom from HOCl increasing by that the abundance of •OH. The reactions of Cl• may lead to formation of undesired chlorinated derivatives and/or promote their oxidation as observed for several aromatic molecules (Mártire et al. 2001; Lei et al. 2021), e.g., in the reaction of Cl• with benzene chlorobenzene forms, albeit with low yield (Alegre et al. 2000).
Cl• reactions are also very important in the atmospheric chemistry. Cl• can form in the cloud droplets by the reactions of Cl– with strongly oxidizing species such as NO3•, SO4•– and •OH (X•) (Buxton et al. 2000; Herrmann 2003). Therefore, Cl– oxidation may represent an important sink of strong oxidants in the tropospheric systems.
In the presence of Cl– in aqueous solutions, Cl• is in equilibrium (7) with Cl2•– with an equilibrium constant of K = 1.4 × 105 mol–1 dm3 (Buxton et al. 1998).
Because of the lack of Cl–, this equilibrium does not exist in organic solvents (e.g., CCl4). Due to the simplicity, many Cl• rate constant measurements, especially in the early period of radical chemistry investigations, were carried out in non-aqueous systems (Alfassi et al. 1989; NDRL, NIST, 2022). These rate constants may differ considerably from the ones determined in aqueous system. Therefore, they are not considered in the present overview.
Equilibrium (7) is established quickly and the reactions of Cl2•– contribute to the degradation of pollutants (Buxton et al. 2000; Mártire et al. 2001). Cl2•– is less reactive than Cl•. Cl• and Cl2•– are oxidants (E(Cl•/Cl–) = 2.4 V and E(Cl2•–/2Cl–) = 2.1 V vs. NHE, Armstrong et al. 2015) and they react with many organic molecules.
Production of Cl• in aqueous solution and methods of rate constant determination
The techniques used for rate constant determination differ in many respects. In some of the techniques, Cl– serves as source of Cl•, in others chlorine containing organic or inorganic compounds are used in Cl• production (chloroacetone, chloramine).
There are two basic techniques for the determination of aqueous-phase rate constants of reactions between radicals and target molecules: the direct and the indirect methods. These techniques have been reviewed and compared in a recent paper published by Ma et al. (2021). When transient techniques (direct method), pulse radiolysis or (laser) flash photolysis are used for rate constant determination, reactive radicals are produced by the energy absorption from a short pulse of accelerated electrons, or (laser) flesh light and the time dependence of transient light absorption of the radical is detected. The kCl• values are determined from the time dependences of the transient absorbance signals. In steady-state experiments (indirect method), mostly relative rate constants are measured (kCl•/kcompetitor) and the absolute value of the compound of interest is calculated by multiplying the relative value by the known rate constant of the competitor. Lei et al. (2019) combined the transient and competitive techniques (see later). A number of rate constants were determined in UV/chlorine process and applying scavenging experiments or complex kinetic modeling to derive kCl•.
In Cl– solutions, most often sulfate radical anions (SO4•–) were used for Cl– oxidation to Cl• (Eq. 8). In radiolytic reactions (Eq. (9)), SO4•– forms in the reaction of hydrated electrons (eaq–) with persulfate anions (S2O82–) (Buxton et al. 1998). In kinetic studies on Cl• reactions, the photolysis of persulfate anions (Eq. (10)) was also often used to generate SO4•– in both transient and steady-state experiments (Zhu et al. 2005; Caregnato et al. 2007; Alegre et al. 2000; Mártire et al. 2001; Ma et al. 2021). Zhu et al. (2019) in their steady-state experiments activated persulfate by Fe2+ ions (11), similar activations were also made using other transient metal ions (e.g., Ti(III), Gilbert et al. 1988).
In Cl– containing systems, the reactions of both Cl• (Eq. (12)) and Cl2•– (Eq. (13)) contribute to the oxidation of the solute molecules (S). At the same time, both radicals react also with the water molecules (Eqs. (14) and (15)):
In the relevant works, complex kinetic models were used to derive kCl• (Buxton et al. 1998, 2001; Alegre et al. 2000; Mártire et al. 2001). Mártire et al. in their time resolved experiments under conditions when Reaction (14) can be neglected described the apparent rate constant of Cl2•– decay by the equation:
K is the equilibrium constant of Reaction (7). Using this technique, first the experimentally determined kapp values were plotted against the solute concentration ([S]) at several constant chloride ion concentrations and the slope values were determined. Then, these slope values obtained at several [Cl–] were plotted as a function of the reciprocal Cl– concentration (1/[Cl–]). The slope of the second plot supplied kCl•.
In another group of experimental techniques used in practice, Cl– was not present in the reaction system, such as when Cl• formed in the photolysis of chloroacetone or chloramine. The photolysis of chloroacetone yields Cl• through the decay of the singlet excited molecule (Buxton et al. 2000; Wicktor et al. 2003; Lei et al. 2019). The participation of the triplet excited cloroacetone molecules was disclosed based on the absence of dissolved oxygen effect:
Cl• reacts relatively slowly with chloroacetone, k18 = 1.0 ± 0.1 × 107 mol–1 dm3 s–1. The chloroacetone concentrations are generally around 10–2 mol dm–3, at this concentration the transient absorption of Cl• (λmax = 320 nm, εmax ≈ 4500 mol–1 dm3 cm–1, Buxton et al. 2000) appears immediately after the pulse. The CH3COCH2• radical has just a small contribution to the absorbance at 320 nm. In case when the transient products do not absorb significantly at the λmax of Cl•, the decay kinetics at 320 nm can be used to determine the rate constant. For molecules with products having observable absorbance beyond the absorbance range of the chlorine atom, the rate constant could be monitored by observing the absorbance of the products formed (Lei et al. 2019; Ma et al. 2021).
The absorption signal of organic radicals formed in (Eq. (12)) for most of compounds overlaps with the absorption of Cl•. Cl•-adducts of benzenes, for example, have absorption maxima at 320–360 nm. In these cases, the competition kinetics method can be used as an alternative in transient experiments. Lei et al. (2019) used chloroacetone to produce Cl•, SCN– as a competitor (Eqs. (19) and (20)). They recorded the absorbances of (SCN)2•– at 472 nm (εmax = 7850 mol–1 dm3 cm–1, Buxton and Stuart 1995) without and with various concentrations of the target compounds.
The competition is described by the following expression:
where A0 is the transient absorbance of [(SCN)2•–] at 472 nm in the absence of S, A is the transient absorbance with S present, [S], [SCN–], [H2O] and [CH3COCH2Cl] are the concentrations of S, SCN–, H2O and CH3COCH2Cl, respectively. At 0.5 and 10 mmol dm–3 SCN– and CH3COCH2Cl concentrations, respectively, G was calculated as 3.6 × 105 s–1. The absorbance (A) of [(SCN)2•–] decreased with increasing [S], and the second-order rate constant was then determined by plotting A0/A against the [S]/(G + k19[SCN–]) ratio.
In several papers, chloramine was used to produce Cl• (Mangalgiri et al. 2019; Sun et al. 2019; Li et al. 2020). In the photolytic process (Eq. (23)), NH2• also forms, this radical is assumed to have low reactivity (Patton et al. 2017):
Recently several authors estimated rate constants based on experiments in the UV/chlorine process. In the experiments used for Cl• rate constant determination, the solutions were spiked with hypochlorous acid/hypochlorite ions (HOCl/OCl−) before the measurements to produce Cl• in Reactions (3) and (4). In these experiments as UV light source low-pressure mercury lamp, UV-LED or solar radiation was used (Sun et al. 2019; Cai et al. 2020; Xiang et al. 2020; Kong et al. 2020; Li et al. 2020; Liu et al. 2020). The UV/chlorine kinetic system is rather complicated with many individual reactions participating (Jin et al. 2011). There are two main radical species present •OH and Cl•. Usually chlorobenzene and/or benzoic acid and nitrobenzene are used to differentiate the reactions of the two reactive radicals. Chlorobenzene and benzoic acid have high rate constants in reaction with both radicals. The reactivity of Cl• with nitrobenzene was suggested to be low (Watts and Linden 2007; Fang et al. 2014; Bulman et al. 2019), while that of with •OH was high. Therefore, it was assumed that, chlorobenzene or benzoic acid reacted with both Cl• and •OH, while nitrobenzene was assumed to react only with •OH (Fang et al. 2014). The rate constants were generally derived using some simulation or fitting procedures. As we will show, this method often gives unrealistic rate constants differing from the values determined by other techniques by more than one order of magnitude. It should be mentioned, that in a recent article Lei et al. (2020) published quite high rate constant (1.01 × 1010 mol–1 dm3 s–1) for the Cl• + nitrobenzene reaction.
Gilbert et al. (1988) applied a special rapid-mixing technique combined with ESR detection for rate constant determination. They produced SO4•– by Ti(III) activation of persulfate ions. The method allowed also the identification of the radicals formed.
Compilation of the published rate constants collected from the original publications is given in the tables. In selecting the compounds for tabulation, we concentrated on molecules of environmental concern. The pH values and the accuracies are indicated as they were published in the original works. Most measurements were made around room temperature; very few temperature dependence studies were published. In the tables, the temperature differing from room temperature is indicated. The tables show also the pKa values of compounds collected from several publications, e.g., Perrin (1965), Babic et al. (2007), Shalaeva et al. (2008). The error bounds in tables represent the σ-level uncertainty. The methods of kCl• determinations are indicated in the tables by abbreviations: PR pulse radiolysis, FP flash photolysis, LFP laser flash photolysis, Comp. competitive method, LFP, C laser flash photolysis combined with the SCN– technique, Compl. and Fit. simulation/modeling/fitting in complex reaction systems often taking into account large numbers of reactions (usually UV/chlorine), Est. estimated based on rate constant of structurally similar compounds, Calc. quantum chemical calculations.
Cl • reaction with inorganic species
In Table 1, we collected a large number of rate constants of Cl• reactions with inorganic molecules and ions. Most of these reactions are important from the point of view of the UV/chlorine system. We mentioned some of these reactions before in connection with the basic chemistry of Cl• and the rate constant measuring techniques. Most of rate constants of reactions with inorganic species in Table 1 are in the 108–109 mol–1 dm3 s–1 range.
The reaction between the chlorine atom and the water molecules (Eq. (14)) is very important from the point of view of the UV/chlorine technique, actually it determines the lifetime of Cl•, and strongly influences the kinetic measurements. As Eqs. (24) and (27) show, in two-step equilibrium processes hydroxyl radical and chloride ion is suggested to be produced in the reaction (Klaning and Wolff 1985; McElroy 1990; Buxton et al. 1998; Yu et al. 2004). However, as McElroy (1990) mentions the experimental observations are not entirely consistent with this mechanism, particularly the apparent absence of any dependence on pH.
The rate constant of Cl• reaction with chloride ion is very high (Eq. (7), 7.8 ± 0.8 × 109 mol–1 dm3 s–1, Yu and Barker 2003), the first step of the dimer radical anion (Cl2•–) formation is followed by several equilibrium reactions, at the end hydroxyl radical can form. At higher pH (above 5) •OH formation is favored. These reactions were detailed in a publication of Buxton et al. (1998), but in our previous review paper on the reactions of Cl2•– with organic molecules of environmental interest we also summarized the mechanism (Wojnárovits and Takács 2021).
In the reactions of Cl• with HOCl and ClO– chlorine monoxide radical (ClO•, E0(ClO•/ClO– = 1.39 V vs. NHE, Armstrong et al. 2015) forms, this radical is a much milder oxidant as Cl•. Therefore, these reactions decrease the oxidizing capacity during the UV/chlorine process (Zehavi and Rabani 1972; Klaning and Wolff 1985). The high rate constant of the Cl• + OH– reaction (1.8 × 1010 mol–1 dm3 s–1, Klaning and Wolff 1985), in which similarly to the reaction with H2O ClOH•– forms, restricts the possibility for the investigations of Cl• reactions to the lower pH region.
A possible combination of UV/chlorine and the Fenton technique is strongly influenced by the high rate constants of the Cl• + H2O2 and Cl• + Fe2+ reactions: 2.0 ± 0.3 × 109 and 1.3 × 1010 mol–1 dm3 s–1, respectively (Yu and Barker 2003; Bjergbakke et al. 1987). The water to be treated always contains some bicarbonate/carbonate ions. Their reactions with Cl• give carbonate radical anions (CO3•–, E0(CO3•–/CO32– = 1.57 V and vs. NHE, Armstrong et al. 2015), a radical anion with lower oxidizing ability and higher selectivity as Cl• (Mertens and von Sonntag 1995).
The rate constants of reactions with most ions (possible impurities in water) take place by single-electron-transfer (SET) mechanism (Buxton et al. 2000).
Simple oxidized molecules
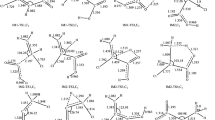
A large number of rate constants are available on Cl• reactions with simple oxidized molecules (Scheme 1) (Gilbert et al. 1988; Mertens and von Sonntag 1995; Buxton et al. 2000; Wicktor et al. 2003): all values are in the 108–109 mol–1 dm3 s–1 range (Table 2). In the experiments of Gilbert et al. (1988), Cl• was generated in the reaction of C1– with SO4•– and H2PO4•– obtained by metal-catalyzed decomposition of the appropriate peroxides. Buxton et al. (2000) and Wicktor et al. (2003) used the laser flash photolysis (LFP) technique for Cl• production in chloroacetone photodecomposition and they calculated the rate constants using the decay of Cl• absorbance. Mertens and von Sonntag (1995) determined the kCl• values in competitive reactions. Minakata et al. (2017) conducted detailed quantum mechanical calculations on the mechanisms. In the case of several molecules, e.g., methanol, ethanol, similar rate constants were measured in two or three laboratories. Generally, the values agreed with each other within one order of magnitude. The authors suggested H-abstraction and Cl-adduct formation as the main mechanisms.
Alcohols
Buxton et al. (2000) and Wicktor et al. (2003) published kCl•=1 × 109 mol–1 dm3 s–1 for methanol (Scheme 1). For alcohols with a higher C-atom number, the values seem to be somewhat bigger. The average of the kCl• values published by Gilbert et al. (1988), Buxton et al. (2000) and Wicktor et al. (2003) for the reaction of ethanol is 2 × 109 mol–1 dm3 s–1. The values published by Mertens and von Sonntag (1995), Buxton et al. (2000) and Wicktor et al. (2003) for 2-propanol are highly different, they are 6 × 109, 1.5 ± 0.1 × 109 and 3.2 ± 0.7 × 109 mol–1 dm3 s–1, respectively. The same is true for tert-butanol: the values of Mertens and von Sonntag (1995) and Buxton et al. (2000) are 3 × 108 and 6.2 ± 0.3 × 108 mol–1 dm3 s–1, respectively, whereas the values of Gilbert et al. (1988) and Wicktor et al. (2003) are 2.2 ± 0.3 × 109 and 1.1 ± 0.1 × 108 mol–1 dm3 s–1, respectively. 1-Propanol and 2-butanol react with kCl• values of 2.2 ± 0.4 × 109 and 5.0 ± 0.6× 109 mol–1 dm3 s–1, respectively (Wicktor et al. 2003).
The H-abstraction from ethanol may take place from both the α and β carbon atom; Gilbert et al. (1988) suggest a 2:1 ratio of the two reactions (bond-strength effect). In the case of tert-butanol, Cl• reacts with the H-atoms on the methyl groups and with the alcoholic OH in 2:1 ratio. The abstraction reaction from OH proceeds with an electron transfer mechanism (a process in which the aqueous environment would be expected to stabilize the incipient charges). The reaction is suggested to proceed in the following way:
The methyl radicals observed support the mechanism.
Aldehydes and ketones
In aqueous solutions aldehydes and ketones undergo hydration, the extent of which strongly depends on the chemical structure. We show this hydration on the example of a ketone:

The hydration does not affect the number of available C-H bonds, but it does introduce O–H groups to the molecule with which Cl• is known to react (Buxton et al. 2000). There may be a relationship between the H• atom abstraction rate constant and the extent of hydration of the carbonyl species. HCHO and CH3CHO are hydrated to ~99 and ~45% extent, respectively, which is reflected also by the significant decrease of the rate constants between formaldehyde and acetaldehyde, 1.4 ± 0.3 × 109 and 6.3 ± 0.4×108 mol–1 dm3 s–1, respectively (Buxton et al. 2000; Wicktor et al. 2003).
The rate constant of 2-butanone is much smaller, 2.4 ± 0.3×108 mol–1 dm3 s–1 (Wicktor et al. 2003), than those measured for formaldehyde and acetaldehyde. For acetone highly different values were published by Buxton et al. (2000) and Wicktor et al. (2003): < 5×106 and 7.8 ± 0.7×107 mol–1 dm3 s–1, respectively.
Acids, acid esters
Buxton et al. (2000) published much smaller rate constant (1.3 ± 0.1 × 108 mol–1 dm3 s–1) for the formic acid + Cl• reaction as determined by Wicktor et al. (2003) (2.8 ± 0.3×109 mol–1 dm3 s–1). The latter authors published high values also for the reactions of propionic and isobutyric acids: 1.2 ± 0.3×109 and 1.7 ± 0.3×109 mol–1 dm3 s–1, respectively. The values published for acetic acid in three laboratories are c.a. one order of magnitude smaller than those of the previously mentioned compounds (Gilbert et al. 1988; Buxton et al. 2001; Wicktor et al. 2003). Otherwise, the dominant reaction mechanism for carboxylic acids and carboxylates, except formic acid and formate, is H-abstraction from a C-H bond. Formate and formic acid undergo Cl-adduct formation predominantly (Minakata et al. 2017).
Simple chloro- and sulfo-compounds
Cl• reacts with chlorinated methanes and chloroacetone (Scheme 2) by H-abstraction reaction (Buxton et al. 1999, 2000, 2001; Wicktor et al. 2003). The rate constant values (107–108 mol–1 dm3 s–1, Table 3) are determined by the C-H bond energy of the attacked bond as in the case of simple oxygenated molecules.
The reactions with the unsaturated compounds, trichloroethylene and tetrachloroethylene take place by Cl• addition to the double bond (Mertens and von Sonntag 1995; Li et al. 2007). The chlorine atom reactions with substituted olefins occur with rate constants values (1.9×108 and 2.8×108 mol–1 dm3 s–1, respectively) smaller by approximately one order of magnitude than those for the OH radical reactions. In the presence of dissolved O2, the Cl• addition reaction is followed by peroxyl radical formation and a short chain reaction starts in which Cl• is the chain carrier released in the bimolecular termination reactions of the various peroxyl radicals formed in the system (Mertens and von Sonntag 1995). In these reactions, highly poisonous phosgene (COCl2) may also form.
Oxidation of dialkyl sulfides in air droplets may play an important role in modifying the global climate since several of their free radical induced oxidation products are water soluble contributing to atmospheric aerosols formation (Zhu et al. 2005). Dimethyl sulfoxide, the slightly oxidized form of dimethyl sulfide reacts with relatively high rate constant of 6.3 ± 0.6×109 mol–1 dm3 s–1, while the reactivities of the more oxidized forms, dimethyl sulfone and methanesulfonate are much smaller, 8.2 ± 1.6 × 105 and 4.9 ± 0.2×105 mol–1 dm3 s–1, respectively. This is a general trend observed in one electron oxidation of sulfur containing molecules: the reactivities of molecules with S-atom in low oxidized state are much higher than those of molecules with high-oxidized S-atom. Zhu et al. (2005) carried out these measurements in laser photolysis experiments in systems where both Cl• and Cl2•– reactive intermediates were present. Transient kinetic spectroscopic measurements in aqueous solutions with dimethyl sulfoxide revealed the formation of chlorine atom–sulfur three electron bonded complexes (Scheme 3). These complexes are generally observed intermediates in one-electron oxidation of sulfur compounds. They are characterized by a sulfur-chlorine three-electron bond with two σ-bonding and one σ*-antibonding electron (Asmus 1990). In a system, where both Cl• and Cl2•– participate in the oxidation the complexes may form in several reactions. However, in carbon tetrachloride solution (no Cl2•–) Sumiyoshi and Katayama (1987) suggested Cl• addition to the S-atom of dimethyl sulfoxide based on spectral and kinetic studies, kCl• = 7.5 ± 0.2×109 mol–1 dm3 s–1.
Simple aromatic molecules
Most of the pharmaceutical, pesticide, personal care, etc., compounds contain benzene ring in their structures. Here, in this chapter we discuss the reactions of Cl• with relatively simple aromatic molecules (Scheme 4). Mártire et al. (2001) reported the same k•Cl values for toluene, chlorobenzene and benzoic acid: 1.8 ± 0.3×1010 mol–1 dm3 s–1 (Table 4). In their former publication, these authors for benzene gave a value of 6.0×109 ≤ kCl• ≤ 1.2×1010 mol–1 dm3 s–1 (Alegre et al. 2000). Watts and Linden (2007) analyzing the reactions in complex aqueous systems containing organic molecules assumed that nitrobenzene reacts with negligible rate with Cl•, at the same time it is highly reactive toward •OH. According to the recent experiments of Lei et al. (2021), the rate constant of the Cl• + nitrobenzene reaction is not negligible, but rather high, 1.01×1010 mol–1 dm3 s–1.
Three mechanisms are considered in Cl• reactions with these simple aromatic molecules: single-electron-transfer (SET) from the ring to Cl•, H-abstraction from the aromatic ring or from the alkyl side chain, and radical addition to the aromatic ring. The SET reaction pathway was disregarded in the cases of benzene, toluene and benzoic acid, since the expected final products in this mechanism, phenol derivatives were not observed (Mártire et al. 2001). Based on the theoretical calculations of Minakata et al. (2017), H-abstraction from the benzene ring is a minor reaction. In the reaction of toluene, the transient absorption spectrum shows sharp bands around 300 nm characteristic to the benzyl type radical. However, as Mártire et al. (2001) mention benzyl type radical may not necessarily form in H-abstraction from the methyl group, but it can also be produced by HCl elimination from an intermediate radical. In the •OH + toluene system, only 6% of •OH produces benzyl radical (Sehested et al. 1975).
Absorption spectra of the transients in laser and conventional flash photolysis experiments (Alegre et al. 2000; Mártire et al. 2001) reveal that Cl• addition to the aromatic rings forming chlorocyclohexadienyl radicals is the predominant mechanism in agreement with the results of theoretical calculations. •OH reacts with aromatic molecules also in radical addition (Homlok et al. 2020). The reaction may occur in one or two steps. In the two-step process, first a π-complex forms: the radical is not stabilized to a single bond, in the second step the radical may stabilize at one of the double bonds giving the σ-complex. In the one-step process, the σ-complex forms directly. In the experiments of Alegre et al. (2000) and Mártire et al. (2001), with the applied time resolution (50 ns) no π-complex was observed. The addition (localization) may take place to any of the carbon atoms in the ring. Some of these radicals are expected to transform to chlorinated stable products in disproportionation reaction. In air saturated solution of benzene chlorobenzene represented less than 10% of the consumed benzene (Alegre et al. 2000).
In the reactions of substituted benzenes (e.g., toluene, benzoic acid), some selectivity in the sites of radical addition is expected, similarly to the reactions of •OH (Homlok et al. 2020). Due to the lack of stereochemical identification of the individual products, the preferred reaction sites are not reported in the relevant works, the authors simply assume, that the observed transient absorption spectra belong to mixtures of the various chlorocyclohexadienyl type radicals. In the reaction of benzoic acid, chlorobenzoic acid isomers and also chlorobenzene were identified as final products. The latter involves a mechanism with COOH→Cl exchange.
In the reaction of chlorobenzene, chlorinated phenols were detected (Mártire et al. 2001): they may form through electron transfer from chlorobenzene to Cl• (SET mechanism). The chlorobenzene cation is suggested to undergo a rapid hydration to hydroxycyclohexadienyl radical. This radical in the subsequent reaction may transform to chlorinated phenols. SET mechanism was proven by Lei et al. (2019) in the Cl• reaction of several substituted aromatic molecules, e.g., 1,3,5-trimethoxybenzene (kCl• ≈ 1.0×1010 mol–1 dm3 s–1). Under acidic conditions, these radical cations were shown to be long-lived enough to be detected by the usual transient kinetics techniques. At neutral and alkaline pH, they undergo very fast decomposition.
In a recent paper, Zhou et al. (2019) estimated the rate constants of reactions of several reactive species (•OH, Cl•, Cl2•– and ClO•) participating in the UV/chlorine process with a series of ionized benzoic acid derivatives at pH 7.2 (3-methyl-, 4-fluoro-, 2-chloro-, 2-iodo- and 3-nitrobenzoate). They set up a kinetic model considering enormously large number of chemical reactions and used fitting procedure to obtain the rate constants. The logarithms of rate constants for all radicals showed good correlations with the Hammett substituent constants with slope values -0.54 (•OH), -2.13 (Cl•), -0.96 (Cl2•–) and -0.45 (ClO•). Based on the slope values, Cl• seems to be a highly selective one-electron oxidant, more selective than, e.g., Cl2•–. However, this suggestion is in disagreement with the general observations (e.g., Lei et al. 2019), in which Cl• seems to be less selective. The kCl• values of Zhou et al. (2019) for the substituted benzoic acids are 1–3 orders of magnitude smaller than the rate constant measured for benzoic acid at similar pH (1.35 ± 0.15×1010 mol–1 dm3 s–1, Lei et al. 2019).
Just the opposite is the case with the rate constant calculated by Li et al. (2021) for 2,4,6-tribromoanisole, 2,4,6-tribromophenol and 2,4,6-tribromophenolate, 7.14×1010, 5.54×1010 and ≥ 2.36×1010 mol–1 dm3 s–1, respectively. The values for the first two are unrealistically high. Moreover, the ionization increases the electron density on the ring. Therefore, higher rate constant is expected for 2,4,6-tribromophenolate than for 2,4,6-tribromophenol.
The rate constants of Cl• reactions with dimethyl-, diethyl and tributyl phthalates (1.8×1010–2.0×1010 mol–1 dm3 s–1, Lei et al. 2019, 2020) agree with the value published for the neutral benzoic acid (1.8×1010 mol–1 dm3 s–1, Mártire et al. 2001). The radical attack is assumed to occur on the aromatic ring.
The rate constants of simple anilines and phenols in Table 4 (aniline, p-toluidine, 4-chloroaniline, phenol, p-cresol, catechol, resorcinol and 4-methylcatechol) are also very high; they are in the 1×1010–4×1010 mol–1 dm3 s–1 range (Li 2017; Lei et al. 2019; Zhang et al. 2022). All values are around the diffusion-controlled limit; this can be the reason that the typical rate enhancing/decreasing effect of the substituents is not observed in the kCl• values. For instance, in spite of the fact that chloroaniline has an electron withdrawing substituent, while p-toluidine has an electron releasing substituent on the ring the rate constants of both molecules are similar to that of aniline.
Molecules with N atom in the aromatic ring generally have low rate constants in radical reactions (Wojnárovits and Takács 2021). That is true also for the Cl• + pyrimidine reaction, which has a kCl• value of 5 ± 1×108 mol–1 dm3 s–1 (Lei et al. 2019). At the same time, the published rate constant values for quinoline (1.2×1010 mol–1 dm3 s–1) caffeine, xanthine and theophylline (~3×1010 mol–1 dm3 s–1) are high.
Pesticides
DEET (N,N-diethyl-m-toluamide) is an often applied insect repellent, it is poorly soluble in water. The published rate constant of reaction with Cl• 3.8×109 mol−1 dm3 s−1 (Sun et al. 2016), was obtained in competitive experiments. It is much smaller than the values published for aniline derivatives. Mecoprop (methylchlorophenoxypropionic acid) is a commonly used herbicide, kCl• = 1.08 × 1010 mol–1 dm3 s–1 (Kong et al. 2020) was reported as measured also in competitive experiments.
Fluconazole and climbazole are used as fungicides (Scheme 5, Table 5). In fluconazole the two electron withdrawing F-atoms decrease the reactivity with the aromatic ring, and the published rate constant, 5.5×109 mol−1 dm3 s−1 (Cai et al. 2020) is smaller than the values of Mártire et al. (2001) published for simple aromatic molecules. The value for climbazole (6.3±1.5×1010 mol−1 dm3 s−1, Cai et al. 2019) is unrealistically high. The rate constant was determined in the UV/free chlorine system, in which both Cl• and •OH were reacting radicals and nitrobenzene was used as scavenger of OH. Atrazine reacts with a much smaller rate constant of 6.87×109 mol–1 dm3 s–1 (Kong et al. 2020). Cl• is expected to abstract H-atom from the alkyl side chains.
The structure of triclosan (fungicide) shows some similarity to that of clofibric acid (discussed later), the high reactivity of triclosan, kCl• = 2.76 ± 0.44×1010 mol−1 dm3 s−1, is probably due to the two aromatic rings in the molecule (Lei et al. 2019).
Antibiotics
The rate constant of amoxicillin, penicillin G and penicillin V, kCl• ≈ 1.2×1010, is much higher, than measured for their structural unit, 6-aminopenicillanic acid, 3.4 ± 0.3×109 mol–1 dm3 s–1, which is responsible for the antibacterial effect (Scheme 6, Table 6) (Lei et al. 2019). The higher value shows, that not the β-lactam part is the main target of Cl• attack. We assume that the main reaction is with the aromatic ring. All the simple aromatic molecules with electron donating substituent have kCl• values above 1×1010 mol–1 dm3 s–1.
7-Aminocephalosporanic acid is regarded as the structural unit, which carries the antibacterial potency of the cephalosporin type β-lactam antibiotics. Its kCl• value, 1.14 ± 0.07×1010 mol–1 dm3 s–1, is close to those of the cephalosporins in Table 6, cefotaxime, cephalexin and cefaclor, ~2×1010 mol–1 dm3 s–1 (Lei et al. 2019). This closeness of the values shows that in cephalosporins the β-lactam part is an important reaction centre in Cl• reaction.
The quinolone antibiotics listed in Table 6 are used in cases of a large number of bacterial infections. Ciprofloxacin, enrofloxacin and ofloxacin have piperazine ring in their structures, they react with Cl with rate constant of ~1.5×1010 mol–1 dm3 s–1 (Lei et al. 2019). The rate constant measured for flumequine, which does not have piperazine ring, is only half of that value (7.7 ± 2.3×109 mol–1 dm3 s–1, Lei et al. 2019). The piperazine ring may show high reactivity toward Cl•.
In the macrolide type antibiotics (azithromycin, erythromycin, roxithromycin), there is a 14 membered macrolide ring, two sugar molecules are linked to this ring. These molecules do not have double bonds or aromatic rings in their structures, susceptible site for Cl• attack. Although these are large molecules, the rate constants are relatively small, for all of them values around 7.5×109 mol–1 dm3 s–1 were published (Lei et al. 2019). For the reactions of dimetridazole and metronidazole, rate constants of ~4.5×109 mol–1 dm3 s–1 were published (Pan et al. 2019; Lei et al. 2019). The similar rate constants for the two nitroimidazole antibiotics suggest that the main site of Cl• attack is the nitroimidazole ring.
The rate constants for the sulfa drugs in Table 6 (sulfanilamide, sulfadimethoxine, sulfadiazine, sulfamethazine, sulfamethoxazole and sulfathiazole) are high; they are in the 3×1010–4×1010 mol–1 dm3 s–1 range (Lei et al. 2019). Sulfapyridine represents an exception, the rate constant is somewhat smaller 8.79 ± 0.27×109 mol–1 dm3 s–1. The latter measurement was made by Liu et al. (2020) by the competitive technique, they also accepted that nitrobenzene does not react with Cl•. At neutral pH, these antibiotics exist in neutral or in anionic forms. In hydroxyl radical reactions, also highly similar rate constants, close to the diffusion controlled limit, were established for all sulfonamides (Wojnárovits et al. 2018). In •OH reaction, detailed final product studies were also conducted with general conclusion that •OH reacts with both the sulfonamide and heterocyclic parts of these molecules. By analogy, Cl• may also react in similar way. Liu et al. (2020) assumed that Cl• mainly attacks the aniline part of these molecules.
2-Phenylbenzimidazole-5-sulfonic acid is a personal care product that is used to protect skin from damage upon solar irradiation. Its chemical structure is similar to those of sulfa drugs. In complex reaction kinetics system, using competitive kinetics, a rate constant of 1.5×1010 mol–1 dm3 s–1 is suggested for its reaction with Cl• (Yin et al. 2022).
Tetracyclines have four hydrocarbon rings in their structures. They are relatively cheap antibiotics used both in human and animal therapy and at subtherapeutic levels as animal growth promoters. The rate constants of both tetracyclines in the table, tetracycline and oxytetracycline are close to the diffusion controlled kCl•.
Trimethoprim as antibiotic mainly used in cases of urinary infections. This medicine is often used in combination with sulfa drugs, e.g., sulfamethoxazole or sulfadiazine. At low pH, both N-atoms in the heterocyclic ring are protonated, at high pH the neutral form dominates (pKa1 3.1, pKa2 7.1). Under the usual conditions, there is a pH dictated equilibrium between the dication (Trim2+), monocation (Trim+) and neutral forms (Wang et al. 2021a). Lei et al. (2019) based on direct and indirect (competitive) measurements at pH 7 and Wu et al. (2016) by estimation suggested kCl• ≈ 2×1010 mol–1 dm3 s–1. The value agrees with the kCl• of 1,3,5-timethoxybenzene, ~2×1010 mol–1 dm3 s–1, this molecule also has three methoxy groups on the benzene ring. Wang et al. (2021a) using the experimental degradation data in the UV/chlorine process and a complex kinetic system in their fitting procedure published, 6.52×109, 3.09×109 and 7.76×109 mol–1 dm3 s–1 rate constant values for the dication, monocation and the neutral molecule, respectively. These values are much smaller than those determined by the previously mentioned authors. We have the feeling that the applied fitting procedure, due to the large number of reactions involved in the reaction system, and the uncertainties in the literature rate constants may have given only an order of magnitude estimate.
Miscellaneous organic compounds
The non-steroidal anti-inflammatory drugs, acetaminophen (paracetamol), aspirin (acetylsalicylic acid), mesalazine (5-aminosalicylic acid), ibuprofen, naproxen and diclofenac (Scheme 7, Table 7) all contain aromatic ring in their structures, a probable part of Cl• attack. The measured rate constants, except for aspirin, are high; they are in the 1×1010–3×1010 mol−1 dm3 s−1 range (Giang et al. 2017; Lei et al. 2019; Li et al. 2020). In a recent paper, by calculations using transient state theory Wang et al. (2021b) published a lower value (2.61×109 mol−1 dm3 s−1) for acetaminophen. This kCl• seems to be unrealistic in view of the measured values, and in view of the high rate constant values published for similar compounds (e.g., simple aromatic molecules). Aspirin has a kCl• of 6.8 ± 1.4×109 mol−1 dm3 s−1. In this molecule, the acetyl group strongly decreases the electron density on the ring. The published rate constant of diclofenac reaction seems to be too high, 3.77 ± 0.65×1010 mol−1 dm3 s−1 (Lei et al. 2019) in view of the two electron withdrawing Cl atoms on one of the rings. However, it should be mentioned that high rate constant at the diffusion-controlled level was also experienced in the reaction of the other strong one-electron oxidant sulfate radical anion (Mahdi Ahmed et al. 2012).
The reactivity of Cl• with atenolol and metoprolol (β blockers) is similar to that of •OH and the basic reaction mechanism is radical addition to the double bonds. Cl• reacts with atenolol and metoprolol with rate constant above 1×1010 mol−1 dm3 s−1 (Mangalgiri et al. 2019; Lei et al. 2019).
Cimitidine and famotidine are used to control stomach acid overproduction. They contain a sulfur atom in the alkyl chain. Based on analogous reactions this S bridge is expected to be the main target in one-electron oxidation (Wojnárovits and Takács 2021). The Cl• rate constants of the two compounds are highly different: 4.3 ± 1.1×109 (cimetidine) and 1.72 ± 0.26×1010 mol−1 dm3 s−1 (famotidine, Lei et al. 2019). Based on the highly different values, we assume that the main place of Cl• attack is not the S-atom, since its surrounding in the chain is the same in both molecules.
For the rate constant of carbamazepine, Wang et al. (2016) published an unrealistically high value of 5.6 ± 1.6×1010 mol–1 dm3 s–1 based on competitive experiments in the complicated carbamazepine-nitrobenzene-benzoic acid system. Under their conditions, both •OH and Cl• reacted with the solutes. Sun et al. (2019) also used the assumption of negligible reaction between Cl• and nitrobenzene and their rate constant value is also high (3.7 ± 0.3×1010 mol–1 dm3 s–1). Lei et al. (2019) in laser flash photolysis experiments found a just bit smaller value, 3.30 ± 0.26×1010 mol–1 dm3 s–1. Li et al. (2017) using rate constant values on similar compounds estimated unrealistically small kCl• of 1.8–3.7×109 mol–1 dm3 s–1.
Clofibric acid (metabolite of several lipid regulators) has a similar reactive part (Cl-Ph-O-R) as climbazole. Lei et al. (2019) published a rate constant of 5.5 ± 1.3×109 mol−1 dm3 s−1 using the SCN− competitive technique in laser flash photolysis experiments for the Cl• + clofibric acid reaction. For this reaction, Lu et al. (2018) published unrealistically high value, 9.76 ± 0.15×1010 mol−1 dm3 s−1. They also used nitrobenzene as probe molecule, assuming that its reaction with Cl• was negligible. The unrealistically high rate constant here also demonstrates that this technique supplies false results. Bezafibrate and gemfibrozil both contain some fragment of clofibric acid. In the former one, there are two aromatic rings (Cl is attached to one of them), in the latter there is one (and no Cl-atom is attached to the aromatic ring), giving explanation for the high rate constants: 1.04 ± 0.09×1010 and 2.14 ± 0.17×1010 mol−1 dm3 s−1, respectively (Lei et al. 2019). Lei et al. (2019) explained the high difference between the rate constants of clofibric acid and gemfibrozil in terms of the presence of two methyl groups on the benzene ring of gemfibrozil and the electron withdrawing chlorine atom on the benzene ring of clofibric acid. The kCl• value of Liu et al. (2021) for gemfibrozil (1.2×109 mol−1 dm3 s−1) obtained in complex kinetic system is unrealistically low compared to the rate constants of compounds with similar structures.
Primidone is an epilepsy medicine. Lei et al. (2019) and Wang et al. (2020) determined highly different kCl• values, 6.2 ± 1.0 × 109 and 3.19 × 1010 mol−1 dm3 s−1, respectively. The latter kCl• was also obtained using nitrobenzene probe molecule.
Due to the three heavy iodine atoms in their structures, iopromide and iohexol are used as X-ray contrast materials in the medical practice. kCl• published for iopromide is high, 2.75±0.39×1010 mol−1 dm3 s−1 (Lei et al. 2019). The value given for iohexol by Zhu et al. (2019) based on modeling calculations is completely unrealistic (1.18 ± 0.22×1012 mol−1 dm3 s−1), it is two orders of magnitude higher than the diffusion controlled limit.
Estrone and estradiol are natural hormones while ethinylestradiol (EE) is an estrogen medication which is widely used in birth control pills. Since estrogens are regularly detected in wastewaters and in natural waters, the mentioned compounds were often used as models in the UV/chlorine process. The rate constants measured by Lei et al. (2019) for the three compounds are ~2.2×1010 mol–1 dm3 s–1; the estimated value of Li et al. (2017) for estradiol (1.3×1010–1.6×1010 mol–1 dm3 s–1) does not differ much from the measured rate constant. However, the rate constant, 2.1 ± 0.2×109 mol–1 dm3 s–1, suggested by Sun et al. (2019) for ethinyl estradiol is certainly unrealistically small. Since the basic structure of the three molecules is similar, we expect not much different rate constants.
Methylparaben (p-hydroxybenzoic acid methyl ester) is an anti-fungal agent often used in a variety of cosmetics and personal-care products. It is also used as a food preservative. The rate constant published for this molecule, 1.52 ± 0.13×1010 mol–1 dm3 s–1 (Lei et al. 2019) is close to the values measured for similar aromatic molecules, e.g., benzoic acid (Table 4). Sucralose is an often used artificial sweetener. Cl• is expected to react with it in H-abstraction reaction: kCl• = 1.11 ± 0.16×1010 mol–1 dm3 s–1 (Lei et al. 2019).
Discussion
Reliability of the methods and the rate constant values
Previously 10–15 different techniques were mentioned used for rate constant determination. For several compounds (e.g., ethanol, tert-butanol, chloroacetone, estradiol, trimethoprim, acetaminophen, naproxen, carbamazepine), two or more rate constants were published eventually obtained in different laboratories by different methods. This fact allows us to say something about the reliability of the applied methods and/or about the obtained rate constant values.
In liquid phase reactions, the rate constants are limited by the diffusion. Buxton et al. (2000) suggested a diffusion controlled rate constant for Cl• reactions around 8.5×109 mol−1 dm3 s−1. Minakata et al. (2017) for the reactions of Cl• with some inorganic ions calculated kdiff values of 1.1×1010 mol−1 dm3 s−1. Lei et al. (2019) in evaluation of their measured values considered a value of ~2×1010 mol−1 dm3 s−1. This value is between the kdiff values suggested for •OH an H•: 1.1×1010 and 2.9×1010 mol−1 dm3 s−1, respectively (Wojnárovits and Takács 2016), and we tend to accept this rate constant as the diffusion controlled limit. Many of the published rate constants approach or even exceed 2×1010 mol−1 dm3 s−1. We suggest to disregard all the rate constants that are much (several times) higher than this value. Such as the rate constant of Wang et al. (2017) for carbamazepine (5.6±1.6 × 1010 mol−1 dm3 s−1) established in calculations using complex kinetic systems. The rate constant in the work of Zhu et al. (2019) on the iohexol reaction (1.18±0.22 × 1012 mol−1 dm3 s−) obtained in modeling calculations is completely unrealistic. Lu et al. (2018) and Cai et al. (2019) used the steady-state competitive technique to determine the kCl• of the clofibric acid and climbazole reactions (9.76±0.15×1010 and 6.3×1010 mol−1 dm3 s−1, respectively). They assumed negligible reaction between applied probe molecule nitrobenzene and Cl•, probably this is the reason of the unrealistically high values. The unrealistic values raise a question on the reliability of the applied techniques.
The values measured by Lei et al. (2019) (SCN– competitive technique, LFP,C) for sulfonamides, 3.12×1010–4.08×1010 mol−1 dm3 s−1, xanthines 3.81×1010–3.98×1010 mol−1 dm3 s−1, diclofenac 3.77×1010 mol−1 dm3 s−1 are also examples of the high values. These authors mention a possible explanation: Cl• may react with these molecules via single electron transfer (SET) reaction, by which the two reactants do not necessarily need to diffuse and encounter in the solvent cage.
In some cases, by using quantum chemical calculations, complex fitting, or modeling procedures too small values were given. For example: the average of four values published for acetaminophen using competitive or product build-up experiments is 1.8 × 1010 mol−1 dm3 s−1 (Giang et al. 2017; Lei et al. 2019; Li et al. 2020), Wang et al. (2021b) based on their calculations reported an order of magnitude smaller value, 2.61×109 mol−1 dm3 s−1. Zhu et al. (2021) gave an order of magnitude lower value for the carbamazepine reaction also as established by other authors.
It is obvious that one has to be rather careful when using a technique for rate constant determination. Especially much care should be taken when Cl• produced in the UV/chlorine process is used in combination with simulation, complex fitting or with competitive (nitrobenzene problem) techniques.
Reaction mechanisms
Very few mechanistic studies were conducted in connection with the Cl• reaction to determine the elementary reaction steps of radical attack on organic molecules, e.g., on identifying the primary organic intermediates. This is because many rate constant determinations were made under steady-state conditions (e.g., using the UV/chlorine processes) which give no possibilities to observe the elementary processes. In the transient kinetic experiments (pulse radiolysis, (laser) flash photolysis) the absorbances of Cl• and Cl2•– complicate the observations of transient organic intermediates (see in the Introduction). However, there are important exceptions, such as 1,3,5-trimethoxybenzene (TMB). In the low pH range, the reaction with Cl• gives radical cation (TMB•+):
This radical cation has a pKa value between pH 3.0 and 5.0 and exhibits strong transient absorbance with λmax = 580 nm (Lei et al. 2019). Based on the intensity of the absorbance, the authors estimated a single-electron-transfer (SET) contribution of 62.4% to the Cl• + TMB reaction. SET also had important contributions to the degradation of some other molecules, such as the sulfonamides. In the transient spectra of these molecules, strong absorptions were observed in the 400–440 nm range, which were attributed to the formation of aniline type radical cations (C6H5NH3•+). Reaction with SET mechanism is suggested also in the reactions of several inorganic ions and alcohols with Cl• (Gilbert et al. 1988). SET reaction pathway was disregarded in the cases of benzene, toluene and benzoic acid, since the expected final products in this mechanism, the phenol derivatives were not observed (Mártire et al. 2001).
The most often suggested reaction between Cl• and organic molecules with unsaturated bonds including also aromatics is the radical addition to the double bond (Minakata et al. 2017). This reaction is similar to the reaction of •OH, but Cl• reactions show less selectivity as those of •OH. Cl• addition to the aromatic ring yields chlorocyclohexadienyl radicals with absorption bands in the 300–350 nm range (Alegre et al. 2000; Mártire et al. 2001; Lei et al. 2019). The aqueous medium seems to stabilize the radical adducts and they decay on much longer timescale as Cl•. This stabilization does not exist in the gas phase (Mártire et al. 2001). In contrast to the non-aqueous systems π-complex formation was not observed in aqueous solutions.
Hydrogen atom abstraction is typical reaction of molecules without double bonds. In the reaction a definite bond-strength effect is observed.
Summary
Rate constants of Cl• reactions were collected from the literature and were discussed together with the methods of determinations and the reaction mechanisms. These rate constants were determined using a large variation of experimental techniques including transient measurements of Cl• decay at λmax = 320 nm (when the absorbances of the starting molecules and the short-lived products do not disturb the observation), or of build-up of the product intermediate at longer wavelengths. Large number of measurements were made in laser flash photolysis experiments utilizing the competition with SCN– ions. In another group of experiments, the rate constants were derived from rather complex reaction systems considering a large number of elementary reactions, using simulations/modeling or fitting procedures and often involving in the determinations radical scavenging experiments. The latter experiments often gave unrealistic rate constant values.
The rate constant values are generally in the 108–109 mol−1 dm3 s−1 range when the basic reaction between Cl• and the target molecule is H-atom abstraction. When the Cl• atom addition to a double bonds dominate the interaction the rate constants are in the 1× 109–2×1010 mol−1 dm3 s−1 range. In the kCl• = 1×1010–4×1010 mol−1 dm3 s−1 range single-electron-transfer reactions may also contribute to the mechanism.
The reactions of Cl• with organic molecules in many respects are similar to the reactions •OH, albeit the chlorine atom seems to be less selective as the hydroxyl radical. However, there is an important difference; in case of •OH single-electron-transfer reactions have minor importance.
Since Cl• atom reactions play very important role in the emerging UV/chlorine technology, some standardization of the rate constant measuring techniques and more kCl• measurements are needed.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Alegre ML, Gerones M, Rosso JA, Bertolotti SG, Braun AM, Mártire DO, Gonzalez MC (2000) Kinetic study of the reactions of chlorine atoms and Cl2•– radical anions in aqueous solutions. 1. Reaction with benzene. J Phys Chem A 104:3117–3125. https://doi.org/10.1021/jp9929768
Alfassi ZB, Mosseri S, Neta P (1989) Reactivities of chlorine atoms and peroxyl radicals formed in the radiolysis of dichloromethane. J Phys Chem 93:1380–1385. https://doi.org/10.1021/j100341a040
Armstrong DE, Huie RE, Koppenol WH, Lymar SV, Merényi G, Neta P, Ruscic B, Stanbury DM, Steenken S, Wardman P (2015) Standard electrode potentials involving radicals in aqueous solution: inorganic radicals (IUPAC Technical Report). Pure Appl Chem 87:1139–1150. https://doi.org/10.1515/pac-2014-0502
Asmus KD (1990) Sulfur-Centered Free Radicals. Methods Enzymol 18:168–180. https://doi.org/10.1016/0076-6879(90)86107-7
Babic B, Horvat AJM, Pavlovic DM, Kastelan-Macan M (2007) Determination of pKa values of active pharmaceutical ingredients. Trends Anal Chem 26:1043–1061. https://doi.org/10.1016/j.trac.2007.09.004
Bjergbakke E, Navaratnam S, Parsons BJ, Swallow AJ (1987) A quantitative description of the action of high-dose pulses of radiation on aerated acid solutions containing ferrous and chloride ions. Radiat Phys Chem 30:59–62. https://doi.org/10.1016/1359-0197(87)90152-4
Bulman DM, Mezyk SP, Remucal CK (2019) The impact of pH and irradiation wavelength on the production of reactive oxidants during chlorine photolysis. Environ Sci Technol 53:4450–4459. https://doi.org/10.1021/acs.est.8b07225
Buxton GV, Stuart CR (1995) Re-evaluation of the thiocyanate dosimeter for pulse radiolysis. J Chem Soc, Faraday Trans 91:279–281. https://doi.org/10.1039/FT9959100279
Buxton GV, Bydder M, Salmon AG (1998) Reactivity of chlorine atoms in aqueous solution Part 1. The equilibrium Cl•+Cl– • Cl2–. J Chem Soc, Faraday Trans 94:653–657. https://doi.org/10.1039/A707377A
Buxton GV, Bydder M, Salmon GA (1999) The reactivity of chlorine atoms in aqueous solution. Transactions on Ecology and the Environment, vol 28. Proceedings of EUROTRAC Symposium '98. Borrell PM, Borrell P (ed), WITPRESS, Southampton, pp. 699–702. https://doi.org/10.2495/EURO991441
Buxton GV, Bydder M, Salmon GA, Williams JE (2000) The reactivity of chlorine atoms in aqueous solution. Part III. The reactions of Cl• with solutes. Phys Chem Chem Phys 2:237–245. https://doi.org/10.1039/A907133D
Buxton GV, Wang J, Salmon GA (2001) Rate constants for the reactions of NO3•, SO4•- and Cl• radicals with formate and acetate esters in aqueous solution. Phys Chem Chem Phys 3:2618–2621. https://doi.org/10.1039/b101932p
Cai W-W, Peng T, Zhang J-N, Hu L-X, Yang B, Yang Y-Y, Chen J, Ying G-G (2019) Degradation of climbazole by UV/chlorine process: Kinetics, transformation pathway and toxicity evaluation. Chemosphere 219:243–249. https://doi.org/10.1016/j.chemosphere.2018.12.023
Cai W-W, Peng T, Yang B, Xu C, Liu Y-S, Zhao J-L, Gu F-L, Ying G-G (2020) Kinetics and mechanism of reactive radical mediated fluconazole degradation by the UV/chlorine process: Experimental and theoretical studies. Chem Eng J 402:126224. https://doi.org/10.1016/j.cej.2020.126224
Caregnato P, Gara PD, Bosio GN, Mártire DO, Gonzalez MC (2007) Reactions of Cl•/Cl2•− radicals with the nanoparticle silica surface and with humic acids: Model reactions for the aqueous phase chemistry of the atmosphere. Photochem Photobiol 83:944–951. https://doi.org/10.1111/j.1751-1097.2007.00087.x
Chen C, Wu Z, Hou S, Wang A, Fang J (2021) Transformation of gemfibrozil by the interaction of chloride with sulfate radicals: Radical chemistry, transient intermediates and pathways. Water Res 209:117944. https://doi.org/10.1016/j.watres.2021.117944
De Laat J, Stefan M (2018) UV/Chlorine process. In: Stefan M (ed.) Advanced Oxidation Processes for Water Treatment. IWA Publishing, London, UK. pp. 383–428. www.iwapublishing.com
Fang J, Fu Y, Shang C (2014) The roles of reactive species in micropollutant degradation in the UV/free chlorine system. Environ Sci Technol 48:1859–1868. https://doi.org/10.1021/es4036094
Giang LT, Phuong DT, Thuy QC, Yen DH (2017) The contribution of free radicals in paracetamol degradation by UV/NaClO. Vietnam J Chem, Internat Ed 55:720–723. https://doi.org/10.15625/2525-2321.2017-00532
Gilbert BC, Stell JK, Peet WJ, Radford KJ (1988) Generation and reactions of the chlorine atom in aqueous solution. J Chem Soc, Faraday Trans 1(84):3319–3330. https://doi.org/10.1039/F19888403319
Grebel JE, Pignatello JJ, Mitch WA (2010) Effect of halide ions and carbonates on organic contaminant degradation by hydroxyl radical-based advanced oxidation processes in saline waters. Environ Sci Technol 44:6822–6828. https://doi.org/10.1021/es1010225
Herrmann H (2003) Kinetics of aqueous phase reactions relevant for atmospheric chemistry. Chem Rev 103:4691–4716. https://doi.org/10.1021/cr020658q
Homlok R, Mile V, Takács E, Járvás G, Sz G, Wojnárovits L (2020) Comparison of hydrogen atom and hydroxyl radical reactions with simple aromatic molecules in aqueous solution. Chem Phys 534:110754. https://doi.org/10.1016/j.chemphys.2020.110754
Huie RE, Clifton CL, Neta P (1991) Electron transfer reaction rates and equilibria of the carbonate and sulfate radical anions. Radiat Phys Chem 38:477–481. https://doi.org/10.1016/1359-0197(91)90065-A
Jayson GG, Parsons BJ, Swallow AJ (1973) Some simple, highly reactive, inorganic chlorine derivatives in aqueous solution. Their formation using pulses of radiation and their role in the mechanism of the Fricke dosimeter. J Chem Soc, Faraday Trans 1 69:1597–1607. https://doi.org/10.1039/F19736901597
Jin J, El-Din MG, Bolton JR (2011) Assessment of the UV/Chlorine process as an advanced oxidation process. Water Res 45:1890–1896. https://doi.org/10.1016/j.watres.2010.12.008
Khanna RK, Armstrong B, Cui H, Tanko JM (1992) Inverted regioselectives in the reactions of chlorine atoms with heteroarylmethanes. J Am Chem Soc 114:6003–6006. https://doi.org/10.1021/ja00041a015
Kishimoto N (2019) State of the art of UV/chlorine advanced oxidation processes: their mechanism, byproducts formation, process variation, and applications. J Water Environ Technol 17:302–335. https://doi.org/10.2965/jwet.19-021
Klaning UK, Wolff T (1985) Laser flash photolysis of HClO, ClO–, HBrO, and BrO– in aqueous solution. Reactions of Cl- and Br-atoms. Ber Bunsenges Phys Chem 89:243–245. https://doi.org/10.1002/bbpc.19850890309
Kong X, Wang L, Wu Z, Zeng F, Sun H, Guo K, Hua Z, Fang J (2020) Solar irradiation combined with chlorine can detoxify herbicides. Water Res 177:115784. https://doi.org/10.1016/j.watres.2020.115784
Lei Y, Cheng S, Luo N, Yang X, An T (2019) Rate constants and mechanisms of the reactions of Cl• and Cl2•– with trace organic contaminants. Environ Sci Technol 53:11170–11182. https://doi.org/10.1021/acs.est.9b02462
Lei Y, Lu J, Zhu M, Xie J, Peng S, Zhu C (2020) Radical chemistry of diethyl phthalate oxidation via UV/peroxymonosulfate process: Roles of primary and secondary radicals. Chem Eng J 379:122339. https://doi.org/10.1016/j.cej.2019.122339
Lei Y, Lei X, Westerhoff P, Zhang X, Yang X (2021) Reactivity of chlorine radicals (Cl• and Cl2•–) with dissolved organic matter and the formation of chlorinated byproducts. Environ Sci Technol 55:689–699. https://doi.org/10.1021/acs.est.0c05596
Li W (2017) Sulfate Radical-Based Advanced Oxidation Treatment for Groundwater Water Treatment and Potable Water Reuse. Dissertation, University of California.
Li K, Stefan MI, Crittenden JC (2007) Trichloroethene degradation by UV/H2O2 advanced oxidation process: Product study and kinetic modeling. Environ Sci Technol 41:1696–1703. https://doi.org/10.1021/es0607638
Li W, Jain T, Ishida K, Liu H (2017) A mechanistic understanding of the degradation of trace organic contaminants by UV/hydrogen peroxide, UV/persulfate and UV/free chlorine for water reuse. Environ Sci - Water Res Technol 3:128–138. https://doi.org/10.1039/C6EW00242K
Li B, Ma X, Deng J, Li Q, Chen W, Li G, Chen G, Wang J (2020) Comparison of acetaminophen degradation in UV-LED-based advanced oxidation processes: Reaction kinetics, radicals contribution, degradation pathways and acute toxicity assessment. Sci Total Environ 723:137993. https://doi.org/10.1016/j.scitotenv.2020.137993
Li M, An Z, Huo Y, Jiang J, Zhou Y, Cao H, He M (2021) Simulation degradation of bromophenolic compounds in chlorine-based advanced oxidation processes: Mechanism, microscopic and apparent kinetics, and toxicity assessment. Chemosphere 291:133034. https://doi.org/10.1016/j.chemosphere.2021.133034
Liu H, Zhang B, Li Y, Fang Q, Hou Z, Tian S, Gu J (2020) Effect of radical species and operating parameters on the degradation of sulfapyridine using UV/chlorine system. Ind Eng Chem Res 59:1505–1516. https://doi.org/10.1021/acs.iecr.9b06228
Liu H, Hou Z, Li Y, Lei Y, Xu Z, Gu J, Tian S (2021) Modeling degradation kinetics of gemfibrozil and naproxen in the UV/chlorine system: Roles of reactive species and effects of water matrix. Water Res 202:117445 s. https://doi.org/10.1016/j.watres.2021.117445
Lu X, Shao Y, Gao N, Chen J, Deng H, Chu W, An N, Peng F (2018) Investigation of clofibric acid removal by UV/persulfate and UV/chlorine processes: Kinetics and formation of disinfection byproducts during subsequent chlor(am)ination. Chem Eng J 331:364–371. https://doi.org/10.1016/j.cej.2017.08.117
Ma J, Minakata D, O’Shea K, Bai L, Dionysiou DD, Spinney R, Xiao R, Wei Z (2021) Determination and environmental implications of aqueous-phase rate constants in radical reactions. Water Res 190:116746. https://doi.org/10.1016/j.watres.2020.116746
Mahdi Ahmed M, Barbati S, Doumenq P, Chiron S (2012) Sulfate radical anion oxidation of diclofenac and sulfamethoxazole for water decontamination. Chem Eng J 197:440–447. https://doi.org/10.1016/j.cej.2012.05.040
Mangalgiri KP, Patton S, Wu L, Xu S, Ishida KP, Liu H (2019) Optimizing potable water reuse systems: Chloramines or hydrogen peroxide for UV-based advanced oxidation process? Environ Sci Technol 53:13323–13331. https://doi.org/10.1021/acs.est.9b03062
Mártire DO, Rosso JA, Bertolotti S, Le Roux GC, Braun AM, Gonzalez MC (2001) Kinetic study of the reactions of chlorine atoms and Cl2•– radical anions in aqueous solutions. II. Toluene, benzoic acid, and chlorobenzene. J Phys Chem A 105:5385–5392. https://doi.org/10.1021/jp004630z
Matthew BM, Anastasio C (2006) A chemical probe technique for the determination of reactive halogen species in aqueous solution: Part 1 – bromide solutions. Atmos Chem Phys 6:2423–2437. https://doi.org/10.5194/acp-6-2423-2
McElroy WJ (1990) A laser photolysis study of the reaction of SO4– with Cl– and the subsequent decay of Cl2– in aqueous solution. J Phys Chem 94:2435–2441. https://doi.org/10.1021/j100369a044
Mertens R, von Sonntag C (1995) Photolysis (λ = 254 nm) of tetrachloroethene in aqueous solutions. J Photochem Photobiol A: Chem 85:1–9. https://doi.org/10.1016/1010-6030(94)03903-8
Minakata D, Kamath D, Maetzold S (2017) Mechanistic insight into the reactivity of chlorine-derived radicals in the aqueous-phase UV–Chlorine advanced oxidation process: Quantum mechanical calculations. Environ Sci Technol 51:6918–6926. https://doi.org/10.1021/acs.est.7b00507
Nagarajan V, Fessenden RW (1985) Flash photolysis of transient radicals. 1. X2- with X = Cl, Br, I, and SCN. J Phys Chem 89:2330–2335. https://doi.org/10.1021/j100257a037
NDRL/NIST Solution Kinetics Database on the Web, https://kinetics.nist.gov/solution/. Last accessed: 2022. 05. 02.
Pan Y, Li X, Fu K, Deng H, Shi J (2019) Degradation of metronidazole by UV/chlorine treatment: Efficiency, mechanism, pathways and DBPs formation. Chemosphere 224:228–236. https://doi.org/10.1016/j.chemosphere.2019.02.081
Patton S, Li W, Couch KD, Mezyk SP, Ishida KP, Liu H (2017) Impact of the ultraviolet photolysis of monochloramine on 1,4-dioxane removal: New insights into potable water reuse. Environ Sci Technol Lett 4:26–30. https://doi.org/10.1021/acs.estlett.6b00444
Perrin DD (1965) Dissociation Constants of Organic Bases in Aqueous Solution. Butterworth, London
Sehested K, Corfitsen H, Christensen HC, Hart EJ (1975) Rates of reaction of O-, OH, and H with methylated benzenes in aqueous solution. Optical spectra of radicals. J Phys Chem 79:310–315. https://doi.org/10.1021/j100571a005
Shalaeva M, Kenseth J, Lombardo F, Bastin A (2008) Measurement of dissociation constants (pKa values) of organic compounds by multiplexed capillary electrophoresis using aqueous and cosolvent buffers. J Pharmacol Sci 97:2581–2606. https://doi.org/10.1002/jps.21287
Shi XT, Liu YZ, Tang YQ, Feng L, Zhang L-Q (2018) Kinetics and pathways of Bezafibrate degradation in UV/chlorine process. Environ Sci Pollut Res 25:672–682. https://doi.org/10.1007/s11356-017-0461-9
Sumiyoshi T, Katayama M (1987) Novel transient absorption of irradiated DMSO in carbon tetrachloride as studied by pulse radiolysis. Chem Lett 16:1125–1126. https://doi.org/10.1246/cl.1987.1125
Sun P, Lee W-N, Zhang R, Huang C-H (2016) Degradation of DEET and caffeine under UV/chlorine and simulated sunlight/chlorine conditions. Environ Sci Technol 50:13265–13273. https://doi.org/10.1021/acs.est.6b02287
Sun P, Meng T, Wang Z, Zhang R, Yao H, Yang Y, Zhao L (2019) Degradation of organic micropollutants in UV/NH2Cl Advanced Oxidation Process. Environ Sci Technol 53:9024–9033. https://doi.org/10.1021/acs.est.9b00749
Thakur SS (2018) Uv/chlorine advanced oxidation processes: factors influencing p-cresol transformation kinetics. MSc Thesis, University of Arizona, http://hdl.handle.net/10150/628152
Wang W-L, Wu Q-Y, Huang N, Wang T, Hu H-Y (2016) Synergistic effect between UV and chlorine (UV/chlorine) on the degradation of carbamazepine: Influence factors and radical species. Water Res 98:190–198. https://doi.org/10.1016/j.watres.2016.04.015
Wang Y, Couet M, Gutierrez L, Allard S, Croué J-P (2020) Impact of DOM source and character on the degradation of primidone by UV/chlorine: Reaction kinetics and disinfection by-product formation. Water Res 172:115463. https://doi.org/10.1016/j.watres.2019.115463
Wang B, Zhang Q, Fu Y, Ran Z, Crittenden JC, Zhang W, Wang H (2021a) Degradation of trimethoprim using the UV/Free chlorine process: Influencing factors and optimal operating conditions. Water 13:1656. https://doi.org/10.3390/w13121656
Wang P, Bu L, Wu Y, Deng J, Zhou S (2021b) Mechanistic insights into paracetamol transformation in UV/NH2Cl process: Experimental and theoretical study. Water Res 194:116938. https://doi.org/10.1016/j.watres.2021.116938
Watts MJ, Linden KG (2007) Chlorine photolysis and subsequent OH radical production during UV treatment of chlorinated water. Water Res 41:2871–2878. https://doi.org/10.1016/j.watres.2007.03.032
Wicktor F, Donati A, Herrmann H, Zellner R (2003) Laser based spectroscopic and kinetic investigations of reactions of the Cl atom with oxygenated hydrocarbons in aqueous solution. Phys Chem Chem Phys 5:2562–2572. https://doi.org/10.1039/B212666D
Wojnárovits L, Takács E (2016) Radiation induced degradation of organic pollutants in waters and wastewaters. Top Curr Chem (z) 374:1–35. https://doi.org/10.1007/s41061-016-0050-2
Wojnárovits L, Toth T, Takács E (2018) Critical evaluation of rate coefficients for hydroxyl radical reactions with antibiotics: a review. Crit Rev Environ Sci Technol 6:575–613. https://doi.org/10.1080/10643389.2018.1463066
Wojnárovits L, Takács E (2021) Rate constants of dichloride radical anion reactions with molecules of environmental interest in aqueous solution. Environ Sci Pollut Res 28:41552–44157. https://doi.org/10.1007/s11356-021-14453-w
Wu Z, Fang J, Xiang Y, Shang C, Li X, Meng F, Yang X (2016) Roles of reactive chlorine species in trimethoprim degradation in the UV/chlorine process: Kinetics and transformation pathways. Water Res 104:272–282. https://doi.org/10.1016/j.watres.2016.08.011
Wu Z, Chen C, Zhu B-Z, Huang C-H, An T, Meng F (2019) Fang J (2019) Reactive nitrogen species are also involved in the transformation of micropollutants by the UV/monochloramine process. Environ Sci Technol 53:11142–11152. https://doi.org/10.1021/acs.est.9b01212
Xiang H, Shao Y, Gao N, Lu X, An N, Chu W (2020) Removal of β-cyclocitral by UV/persulfate and UV/chlorine process: Degradation kinetics and DBPs formation. Chem Eng J 382:122659. https://doi.org/10.1016/j.cej.2019.122659
Yin K, Li T, Zhang T, Zhang Y, Yang C, Luo S (2022) Degradation of organic filter 2-Phenylbenzidazole-5-Sulfonic acid by light-driven free chlorine process: Reactive species and mechanisms. Chem Eng J 430:132684. https://doi.org/10.1016/j.cej.2021.132684
Yu X-Y, Barker JR (2003) Hydrogen peroxide photolysis in acidic aqueous solution containing chloride ions. I Chemical Mechanism. J Phys Chem A 107:1313–1324. https://doi.org/10.1021/jp0266648
Yu X-Y, Bao Z-C, Barker JR (2004) Free radical reactions involving Cl•, Cl2•–, and SO4•– in the 248 nm photolysis of aqueous solutions containing S2O82– and Cl–. J Phys Chem A 108:295–308. https://doi.org/10.1021/jp036211i
Zehavi D, Rabani J (1972) The oxidation of aqueous bromide ions by hydroxyl ions and hydroxyl radicals. Pulse Radiolytic Investigation J Phys Chem 76:312–319. https://doi.org/10.1021/j100647a006
Zhang W, Zhou S, Sun J, Meng X, Luo J, Zhou D, Crittenden J (2018) Impact of chloride ions on UV/H2O2 and UV/persulfate advanced oxidation processes. Environ Sci Technol 52:7380–7389. https://doi.org/10.1021/acs.est.8b01662
Zhang X, He J, Lei Y, Qui Z, Cheng S, Yang X (2019) Combining solar irradiation with chlorination enhances the photochemical decomposition of microcystein-LR. Water Res 159:324–332. https://doi.org/10.1016/j.watres.2019.05.030
Zhang X, Zhai J, Lei Y, Huang H, Ren P, Lambropoulou D, Yang X (2022) Enhanced formation of trichloronitromethane precursors during UV/monochloramine treatment. J Hazard Mater 422:126813. https://doi.org/10.1016/j.jhazmat.2021.126813
Zhou S, Zhang W, Sun J, Zhu S, Li K, Meng X, Luo J, Shi Z, Zhou D, Crittenden JC (2019) Oxidation mechanisms of the UV/free chlorine process: kinetic modelling and quantitative structure activity relationships. Environ Sci Technol 53:4335–4345. https://doi.org/10.1021/acs.est.8b06896
Zhu L, Nicovich JM, Wine PH (2005) Kinetics studies of aqueous phase reactions of Cl atoms and Cl2•– radicals with organic sulfur compounds of atmospheric interest. J Phys Chem A 109:3903–3911. https://doi.org/10.1021/jp044306u
Zhu J-P, Li Y-L, Zhang T-Y, Cao T-C, Xu B, Pan Y, Zhang X-T, Gao N-Y (2019) Modelling of iohexol degradation in a Fe(II)-activated persulfate system. Chem Eng J 367:86–93. https://doi.org/10.1016/j.cej.2019.02.120
Zhu S, Tian Z, Wang P, Zhang W, Bu L, Wu Y, Dong B, Zhou S (2021) The role of carbonate radicals on the kinetics, radical chemistry, and energy requirement of UV/chlorine and UV/H2O2 processes. Chemosphere 278:130499. https://doi.org/10.1016/j.chemosphere.2021.130499
Acknowledgements
This work was supported by the National Office for Research and Development through the Hungarian-Chinese Industrial Research and Development Cooperation Project (No. 2017-2.3.6.-TÉT-CN-2018-00003). The Chinese authors acknowledged the financial support of the Key Program for Intergovernmental S&T Innovative Cooperation Project (2017YFE0127000).
Funding
Open access funding provided by Centre for Energy Research. Partial financial support was received from the National Office for Research and Development through the Hungarian-Chinese Industrial Research and Development Cooperation Project (No. 2017–2.3.6.-TÉT-CN-2018–00003).
Author information
Authors and Affiliations
Contributions
László Wojnárovits: Conceptualization; Writing—original draft.
Jianlong Wang: Writing—review.
Libing Chu: Writing—review.
Erzsébet Takács: Writing—original draft, Writing—review & editing.
All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Responsible Editor: George Z. Kyzas
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
- Rate constants of Cl• reactions show large scatter depending on the method used
- Fitting or simulation in UV/chlorine process is not suitable for kCl• determination
- Cl• shows higher reactivity and lower selectivity as •OH
- In Cl• reactions, SET has higher role than in •OH reactions
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wojnárovits, L., Wang, J., Chu, L. et al. Rate constants of chlorine atom reactions with organic molecules in aqueous solutions, an overview. Environ Sci Pollut Res 29, 55492–55513 (2022). https://doi.org/10.1007/s11356-022-20807-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-20807-9