Abstract
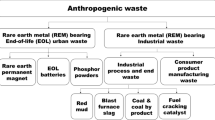
Germanium as a strategic metalloid is widely used in high-tech devices. The most crucial germanium resources are rare and limited to zinc minerals, i.e., especially zinc sulfides and coal-related products. Other than coals and zinc minerals, materials such as WEEEs (wastes from electrical and electronic equipment) and catalysts are considered secondary resources of germanium. Since there is no specific mineral for germanium, it should be extracted from the resources above as a by-product. Primary resources contribute to 70% of germanium production, whereas the rest is produced from recycled materials. The world refinery production of germanium enhanced by about 7% in 2020 compared to 2019. This growing demand for germanium encourages the industry to find other resources and extraction technologies. Germanium can be recovered after leaching of different resources in acidic, water, or alkaline media followed by processing using various hydro/pyrometallurgical methods. Several reviews and articles have been published to review the resources and processes for the germanium separation. However, no one did not present a final road map from feasibility views and environmental aspects. This review proposes a road map for germanium recycling based on the performance and economic availability, process efficiency, operational issues, and process feasibility.







Similar content being viewed by others
Data availability
Not applicable.
Code availability
It is not applicable.
Abbreviations
- Aliquat 336:
-
A quaternary ammonium salt
- AMBERSEP™ IRA743:
-
A unique macroporous, weak base anion resin
- AMBERLITE™ IRA900 Cl:
-
A macro reticular polystyrene strong base anion exchange resin containing quaternary ammonium groups in styrene–divinylbenzene copolymer matrix
- AMBERLITE™ IRA958 Cl:
-
A macro reticular strongly basic anion exchange resin having quaternary ammonium functionality in a cross-linked acrylic polymer matrix
- Cat/CAT:
-
Catechol
- Cit:
-
Citric
- CTA:
-
Cetyl trimethyl ammonium
- CTAB:
-
Cetyl trimethyl ammonium bromide
- Cyanex 301:
-
Dialky1-dithiophosphinic acid
- Cyanex 923:
-
A liquid phosphine oxide
- Cyphos IL 104:
-
Trihexyl(tetradecyl)phosphonium bis-2,4,4-(trimethylpentyl)phosphinate
- D2EHPA:
-
Di-(2-Ethylhexyl) phosphoric acid
- D403:
-
Chelating resin with N-methylglucamine groups
- DOMPA:
-
Dioctyl ethylene bis phosphonic acid
- WEEE:
-
Waste from electrical and electronic equipment
- EG:
-
Ethylene glycol
- FSSLM:
-
Flat sheet supported liquid membrane
- GCFA:
-
Gasification coal fly ashes
- H106:
-
Hydroxamic acids
- HF:
-
Hollow fiber
- IGCC:
-
Integrated gasification combined cycle
- Ionquest 801:
-
2-Ethylhexyl phosphonic acid mono-2-ethylhexyl ester
- Kelex 100:
-
7-(4-Ethyl-l-methyl-octyl)-8-hydroxyquin
- L:S:
-
Liquid to solid ratio
- LED:
-
Light-emitting diode
- LIX 26:
-
Alkyl substituted 8-hydroxyquinoline
- LIX 63:
-
5,8-Diethyl-7-hydroxydodecan-6-oxime
- N235:
-
Tri (octyl–decyl) amine
- OPAP:
-
Octyl phenyl acid phosphate
- RX-1:
-
An anion-exchange membrane
- S:L:
-
Solid to liquid ratio
- ShelFlSol 2046:
-
A special Kerosene-cut with a mixture of paraffins, naphthenes, and aromatics
- T:
-
Tartaric
- TBP:
-
Tri butyl phosphate
- TOA:
-
Tri octyl amine
- TOP:
-
Tri octyl phosphate
- TOPO:
-
Tri octyl phosphine oxide
- YW100:
-
Unknown
References
Abgrall N et al (2018) The processing of enriched germanium for the Majorana Demonstrator and R&D for a next generation double-beta decay experiment. Nucl Instrum Methods Phys Res Sect A 877:314–322
Andrianov AM, Avlasovich LM (1968) Extraction of germanium by tri-n-octylamine. Zh Prikl Khim 41:2313
Anonymous (1997) 10 - Germanium, Tin and Lead. In: Greenwood NN, Earnshaw A (eds) Chemistry of the Elements (Second Edition). Butterworth-Heinemann, Oxford, pp 367–405
Arrambide Cruz C, Marie S, Arrachart G, Pellet-Rostaing S (2018) Selective extraction and separation of germanium by catechol based resins. Sep Purif Technol 193:214–219
Arroyo F, Fernández-Pereira C (2008) Hydrometallurgical recovery of germanium from coal gasification fly ash. Solvent extraction method. Ind Eng Chem Res 47:3186–3191
Arroyo F, Fernández-Pereira C, Olivares J, Coca P (2009a) Hydrometallurgical recovery of germanium from coal gasification fly ash: pilot plant scale evaluation. Ind Eng Chem Res 48:3573–3579
Arroyo F, Font O, Fernández-Pereira C, Querol X, Juan R, Ruiz C, Coca P (2009c) Germanium recovery from gasification fly ash: evaluation of end-products obtained by precipitation methods. J Hazard Mater 167:582–588
Arroyo F, Fernández-Pereira C (2009) Method for the recovery of germanium in solution by means of complexing and use of ion-exchange resins, WO2009/106660, WO Patent, the World Intellectual Property Organization
Arroyo F, Camacho NP, Coca P, Fernández-Pereira C (2009a) Recovery of germanium from coal fly ash leachate by precipitation. The World of Coal Ash Proceeding, Lexington, Kentucky, USA
Arroyo F, Font O, Fernandez-Pereira C (2009c) Germanium and gallium extraction from gasification fly ash: optimization for up-scaling a recovery process. The World of coal ash conference, Lexington, Kentucky, USA
Besedin A (2017) Market research, global market for germanium and germanium products. Independently Published, United Kingdom
Boateng DA, Neudorf DA, Saleh VN (1990) Recovery of germanium from aqueous solutions by solvent extraction. US4915919A, US Patents. The United States Patent and Trademark Office
Brinkman WF, Haggan DE, Troutman WW (1997) A history of the invention of the transistor and where it will lead us. IEEE J Solid-State Circuits 32:1858–1865
Bruckner M, de Schutter L, Schriefl E, Haider A, Bohunovsky L, Lugschitz B, Polzin C (2013) Feasible futures for the common good. energy transition paths in a period of increasing resource scarcities progress report 3: scenarios of RE technology growth. Sustainable Europe Research Institute (SERI), energieautark consulting gmbh, Vienna, Austria
Buban KR, Collins MJ, Masters IM, Trytten LC (2013) Comparison of direct pressure leaching with atmospheric leaching of zinc concentrates. Lead-Zinc 2000 proceeding, Pittsburgh, USA, pp 727–738
Chaturabul S, Srirachat W, Wannachod T, Ramakul P, Pancharoen U, Kheawhom S (2015) Separation of mercury (II) from petroleum produced water via hollow fiber supported liquid membrane and mass transfer modeling. Chem Eng J 265:34–46
Chen W-S, Chang B-C, Chiu K-L (2017) Recovery of germanium from waste optical fibers by hydrometallurgical method. J Environ Chem Eng 5:5215–5221
Chen W-S, Chang B-C, Shuai C-k (2020) Improve subsequent leaching efficiency and extraction rate of germanium in optical fibre cables with pre-treatment. IOP Conference Series: Mater Sci Eng 720:012005
Chen W-S, Chang B-C, Chen Y-J (2018) Using ion-exchange to recovery of germanium from waste optical fibers by adding citric acid. In: IOP earth and environmental science conference proceeding, p 012008
Chirkst D, Chistyakov A, Cheremisina O, Zhadovskii I (2008) Sorption of germanium from alkaline solutions on anion-exchange resin. Russ J Appl Chem 81:38–41
Cote G, Bauer D (1980) Liquid—liquid extraction of germanium with oxine derivatives. Hydrometallurgy 5:149–160
Curtolo DC, Friedrich S, Friedrich B (2017) High purity germanium, a review on principle theories and technical production methodologies. J Cryst Proc Technol 7:20
Daocheng L (2007) Leaching Germanium from low-grade lignite containing germanium with microorganism [J]. Coal Chemical Industry 4:016
De Schepper A (1976) Liquid-liquid extraction of germanium by LIX 63. Hydrometallurgy 1:291–298
De Schepper A, Coussement M, Van Peteghem A (1984) Process for separating germanium from an aqueous solution by means of an alphahydroxyoxime. US4432952A, US Patent. The United States Patent and Trademark Office
Dhiman S, Gupta B (2020) Recovery of pure germanium oxide from Zener diodes using a recyclable ionic liquid Cyphos IL 104. J Environ Manage 276:111218
Drzazga M, Prajsnar R, Chmielarz A, Benke G, Leszczyńska-Sejda K, Ciszewski M, Bilewska K, Krawiec G (2018) Germanium and indium recovery from zinc metallurgy by-products—dross leaching in sulphuric and oxalic acids. Metals 8:1041
Drzazga M, Chmielarz A, Benke G, Leszczyńska-Sejda K, Knapik M, Kowalik P, Ciszewski M (2019) Precipitation of germanium from sulphate solutions containing tin and indium using tannic acid. Appl Sci 9:966
Drzazga M, Benke G, Ciszewski M, Knapik M, Radoń A, Kozłowicz S, Goc K, Kowalik P, Leszczyńska-Sejda K (2020) Recovery of germanium from sulphate solutions containing indium and tin using cementation with zinc powder. Minerals 10:358
Drzazga M, Palmowski A, Benke G, Ciszewski M, Leszczyńska-Sejda K (2021) Recovery of germanium and indium from leaching solution of germanium dross using solvent extraction with TOA, TBP and D2EHPA. Hydrometallurgy 202:105605
Efremov V, Potolokov V, Nikolashin S, Fedorov V (2002) Chemical equilibria in hydrolysis of germanium tetrachloride and arsenic trichloride. Inorg Mater 38:847–853
Epouse Bauer, Rouillard D, Cote G, Fossi P, Marchon B (1983) Process for selective liquid-liquid extraction of germanium. US4389379A, US Patents. The United States Patent and Trademark Office
Everest DA, Harrison JC (1960) The chemistry of quadrivalent germanium. Part VIII. Complexes of germanium with tartaric, lactic, and mucic acid. J Chem Soc (Resumed) 0:3752–3758
Fassbender M (2007) Separation of germanium-68 from gallium-68. US20070207075A1, US Patents. The United States Patent and Trademark Office
Fleitlikh IY, Grigorieva NA, Logutenko OA (2021) Extraction behavior of germanium in Kelex 100 and LIX 63 systems. ChemistrySelect 6:4285–4291
Fogg HC, James C (1919) The extraction of gallium and germanium from zinc oxide. J Am Chem Soc 41:947–949
Font O, Querol X, Huggins FE, Chimenos JM, Fernández AI, Burgos S, Peña FG (2005) Speciation of major and selected trace elements in IGCC fly ash. Fuel 84:1364–1371
Font O, Querol X, López-Soler A, Chimenos JM, Fernández AI, Burgos S, García Peña F (2005) Ge extraction from gasification fly ash. Fuel 84:1384–1392
Frenzel M, Mikolajczak C, Reuter MA, Gutzmer J (2017) Quantifying the relative availability of high-tech by-product metals — the cases of gallium, germanium and indium. Resour Policy 52:327–335
Ft AT, Fernández-Pereira C, MaC C (2010) Recovery of germanium from aqueous solutions by ion-exchange extraction of its catechol complex. Ind Eng Chem Res 49:4817–4823
Glockling F (1969) Chemistry of Germanium. Sci Gov Rep 164:1160
Grdenić D, Jagodić V (1964) Separation of germanium from arsenic by solvent extraction with dioctyl methylenebisphosphonic acid. J Inorg Nucl Chem 26:167–170
Grigorieva NA, Kulmuchamedov GK, Fleitlikh IY (2020) Germanium extraction from sulfuric acid solutions in the presence of thiocyanate ion. J Siberian Federal Univ Chem 13:542–552
Gupta B, Mudhar N (2006) Extraction and separation of germanium using Cyanex 301/Cyanex 923. Its recovery from transistor waste. Sep Sci Technol 41:549–572
Habashi F (1997) Hand Book of Extractive Metallurgy, 1. Wiley-VCH
Habashi F (1997) Handbook of Extractive Metallurgy, 3. Wiley-VCH, Weinheim
Haller EE (2006) Germanium: from its discovery to SiGe devices. Mater Sci Semicond Process 9:408–422
Harbuck DD, Judd JC, Behunin DV (1991) Germanium solvent extraction from sulfuric acid solutions (and co-extraction of germanium and gallium). Solvent Extr Ion Exch 9:383–401
Harbuck DD (1992) Report of Investigations on Gallium and germanium recovery from domestic sources. US Department of the Interior, Bureau of Mines
Höll R, Kling M, Schroll E (2007) Metallogenesis of germanium—a review. Ore Geol Rev 30:145–180
Immanuel AA, Ewalt TC (1960): Process for germanium recovery. US2929677A, US Patents. The United States Patent and Trademark Office
Ingri N (1963) Equilibrium studies of polyanions 12. Polygermanates in Na(Cl) medium. Acta Chem. Scand.: 597–616
Inukai Y, Kaida Y, Yasuda S (1997) Selective separation of germanium(IV) by iminodiacetic acid-type chitosan chelating resin. Anal Sci 13:339–344
Inukai Y, Chinen T, Matsuda T, Kaida Y, Yasuda S (1998) Selective separation of germanium (IV) by 2, 3-dihydroxypropyl chitosan resin. Anal Chim Acta 371:187–193
Inukai Y, Tanaka Y, SHIRAISHI Y, Matsuda T, Mihara N, Yamada K, Nambu N, Itoh O, Doi T, Kaida Y (2002) Selective separation of germanium (IV) by di (2-hydroxyethyl) amine-type cellulose derivative. In: Analytical Sciences/Supplements Proceedings of IUPAC International Congress on Analytical Sciences 2001 (ICAS 2001), The Japan Society for Analytical Chemistry, Tokyo, Japan, pp i1117–i1120
Jiang T, Zhang T, Ye F, Liu Z (2019) Occurrence state and sulfuric-acid leaching behavior of germanium in secondary zinc oxide. Miner Eng 137:334–343
Jiang T, Zhang T, Liu Z (2020) Recovery of Germanium via H2SO4/MnO2 Leaching–NaAc leaching/Na2CO3 precipitation–tri(octyl-decyl) amine stepwise solvent extraction. ACS Sustain Chem Eng 8:18545–18557
Jiang T, Zhang T, Liu Z (2021) Pb-based aggregate, Ge-galena coexistence, and Ge-anglesite coprecipitate—Limitations and an improvement of germanium recovery from secondary zinc oxide via H2SO4 leaching. Hydrometallurgy 200:105543
Johnson OH (1952) Germanium and its inorganic compounds. Chem Rev 51:431–469
Kamran Haghighi H, Irannajad M, Fortuny A, Sastre AM (2018) Recovery of germanium from leach solutions of fly ash using solvent extraction with various extractants. Hydrometallurgy 175:164–169
Kamran Haghighi H, Irannajad M, Fortuny A, Sastre AM (2018) Mathematical modeling for facilitated transport of Ge (IV) through supported liquid membrane containing Alamine 336. Chem Pap 72:955–970
Kamran Haghighi H, Irannajad M, Fortuny A, Sastre AM (2019) Selective separation of Germanium(IV) from simulated industrial leachates containing heavy metals by non-dispersive ionic extraction. Article in Press, Miner. Eng
Kamran Haghighi H, Irannajad M, Maria Sastre A (2019) Germanium transport across supported liquid membrane with Cyanex 923: mathematical modeling. Transactions of Nonferrous Metals Society of China 29:1956–1966
Kamran Haghighi H, Irannajad M, Teresa Coll M, Sastre A (2019) Non-dispersive extraction of Ge (IV) from aqueous solutions by Cyanex 923: transport and modeling studies. Metals 9:676
Kamran Haghighi H, Irannajad M, Moradkhani D (2018) Permeation and modeling studies on Ge(IV) facilitated transport using trioctylamine through supported liquid membrane. Korean J Chem Eng 35:53–60
Kamran Haghighi H, Irannajad M, Fortuny A, Sastre AM (2019a) Non-dispersive selective extraction of germanium from fly ash leachates using membrane-based processes. Sep Sci Technol 54:2879–2894
Kamran Haghighi H, Irannajad M, Moradkhani D (2019d) Facilitated transport of germanium from acidic medium through supported liquid membrane using Cyanex 301 as mobile carrier. Physicochem Probl Min Process 55:225–236
Kamran Haghighi H Irannajad M (2018) Study of effective parameters on the performance of supported liquid membranes in the extraction of germanium. Ph.D. Thesis. Department of Mining Engineering, Amirkabir University of Technology, Tehran, Iran
Kul M, Topkaya Y (2008) Recovery of germanium and other valuable metals from zinc plant residues. Hydrometallurgy 92:87–94
Kuroiwa K, Ohura S-i, Morisada S, Ohto K, Kawakita H, Matsuo Y, Fukuda D (2014) Recovery of germanium from waste solar panels using ion-exchange membrane and solvent extraction. Miner Eng 55:181–185
Lapidus AL, Khudyakov DS, Beilina NY, Trukhina MA, Kozlov AM, Zhagfarov FG (2022) Solid fossil fuels as a source of trace elements. Solid Fuel Chem 56:1–14
Lebleu A, Fossi P, Demarthe J-M (1978): Process for the recovery and purification of germanium from zinc ores. US4090871A, US Patents. The United States Patent and Trademark Office
Liang D, Wang J, Wang Y (2008) Germanium recovery by co-precipitation of germanium and iron in conventional zinc metallurgy. J Southern Afr Inst Mining Metall 108:1–10
Liang D, Wang J, Wang Y (2009) Difference in dissolution between germanium and zinc during the oxidative pressure leaching of sphalerite. Hydrometallurgy 95:5–7
Liang J, Fan L, Xu K, Huang Y (2012) Study on extracting of germanium with trioctylamine. Energy Procedia 17:1965–1973
Licht C, Peiró LT, Villalba G (2015) Global substance flow analysis of gallium, germanium, and indium: quantification of extraction, uses, and dissipative losses within their anthropogenic cycles. J Ind Ecol 19:890–903
Liu F, Yang Y, Lu Y, Shang K, Lu W, Zhao X (2010) Extraction of germanium by the AOT microemulsion with N235 system. Ind Eng Chem Res 49:10005–10008
Liu F, Liu Z, Li Y, Liu Z, Li Q, Zeng L (2016) Extraction of gallium and germanium from zinc refinery residues by pressure acid leaching. Hydrometallurgy 164:313–320
Liu F, Liu Z, Li Y, Wilson BP, Liu Z, Zeng L, Lundström M (2017) Recovery and separation of gallium(III) and germanium(IV) from zinc refinery residues: Part II: Solvent extraction. Hydrometallurgy 171:149–156
Liu F, Liu Z, Li Y, Wilson BP, Lundstrom M (2017) Behavior of gallium and germanium associated with zinc sulfide concentrate in oxygen pressure leaching. Physicochem Probl Miner Process 53:1047–1060
Liu F, Liu Z, Li Y, Wilson BP, Lundström M (2017c) Recovery and separation of gallium(III) and germanium(IV) from zinc refinery residues: part I: leaching and iron(III) removal. Hydrometallurgy 169:564–570
Ma X-h, Qin W-q, Wu X-l (2013) Extraction of germanium(IV) from acid leaching solution with mixtures of P204 and TBP. J Cent South Univ 20:1978–1984
Marchon B, Cote G, Bauer D (1979) Some typical behaviours of the β-dodecenyl 8-hydroxyquinoline through its reactions with germanium(IV). J Inorg Nucl Chem 41:1353–1363
Marco-Lozar JP, Cazorla-Amorós D, Linares-Solano A (2007) A new strategy for germanium adsorption on activated carbon by complex formation. Carbon 45:2519–2528
Martsinko EE, Seifullina II, Minacheva LK, Pesaroglo AG, Sergienko VS (2008) Synthesis, properties, and molecular and crystal structure of diantipyrylmethanium Bis(μ-tartrato)dihydroxydigermanate(IV) tetrahydrate (HDAm)2[Ge2(μ-L)2(OH)2] · 4H2O. Russ J Inorg Chem 53:1694–1702
Morgan G, Davies GR (1937) Germanium and gallium in coal ash and flue dust. J Soc Chem Ind 56:717–721
Moskalyk RR (2004) Review of germanium processing worldwide. Miner Eng 17:393–402
Nazarenko VA (1974) Analytical Chemistry of Germanium. Wiley, New York
Newton DE (2010) Chemical Elements. UXL Publisher, Farmington Hills, Michigan, The United States
Nusen S, Zhu Z, Chairuangsri T, Cheng CY (2015) Recovery of germanium from synthetic leach solution of zinc refinery residues by synergistic solvent extraction using LIX 63 and Ionquest 801. Hydrometallurgy 151:122–132
Ozawa I, Saito K, Sugita K, Sato K, Akiba M, Sugo T (2000) High-speed recovery of germanium in a convection-aided mode using functional porous hollow-fiber membranes. J Chromatogr A 888:43–49
Park H-J, Tavlarides LL (2009) Germanium (IV) adsorption from aqueous solution using a Kelex-100 functional adsorbent. Ind Eng Chem Res 48:4014–4021
Pokrovski GS, Schott J (1998) Experimental study of the complexation of silicon and germanium with aqueous organic species: implications for germanium and silicon transport and Ge/Si ratio in natural waters. Geochim Cosmochim Acta 62:3413–3428
Pokrovski GS, Martin F, Hazemann J-L, Schott J (2000) An X-ray absorption fine structure spectroscopy study of germanium-organic ligand complexes in aqueous solution. Chem Geol 163:151–165
Pokrovsky OS, Pokrovski GS, Schott J, Galy A (2006) Experimental study of germanium adsorption on goethite and germanium coprecipitation with iron hydroxide: X-ray absorption fine structure and macroscopic characterization. Geochim Cosmochim Acta 70:3325–3341
Powell A, Lever F, Walpole R (1951) The extraction and refining of germanium and gallium. J Chem Technol Biotechnol 1:541–551
Rafiee P, Ghassa S, Moosakazemi F, Khosravi R, Siavoshi H (2021) Recovery of a critical metal from electronic wastes: germanium extraction with organic acid. J Clean Prod 315:128223
Rao S, Wang D, Liu Z, Zhang K, Cao H, Tao J (2019) Selective extraction of zinc, gallium, and germanium from zinc refinery residue using two stage acid and alkaline leaching. Hydrometallurgy 183:38–44
Rochow EG, Abel EW (2014) The chemistry of germanium: tin and lead, 14. Elsevier
Ruiz AG, Sola PC, Palmerola NM (2018) Germanium: current and novel recovery processes. Adv Mater Device App Germanium 1:10–29
Sargar BM, Anuse MA (2005) Solvent extraction separation of germanium(IV) with N-n-octylaniline as an extractant. J Anal Chem 60:404–408
Shikun P, Yaozhong L, Yanjun L (2013) Recovery Ge from pulverized fuel ash through the method of H2SO4-NH4F-NaClO3 - leaching-tannin precipitation. Advanced Materials Research 669:377–383
Shimanskii A, Yasinskiy A, Yakimov I, Losev V, Buyko O, Malyshkin A, Kazantsev Y (2021) Aluminum smelting carbon dust as a potential raw material for gallium and germanium extraction. JOM 73:1103–1109
Song J, Peng C, Liang Y, Zhang D, Lin Z, Liao Y, Wang G (2021) Efficient extracting germanium and gallium from zinc residue by sulfuric and tartaric complex acid. Hydrometallurgy 202:105599
Stroganova EA, Bezryadin SG, Larina TV (2019) The equilibrium of germanium(IV) and copper(II) ions sorption from chloride solutions on the anion-exchange resin AN-31. Adsorption 26:349–359
Takemura H, Morisada S, Ohto K, Kawakita H, Matsuo Y, Fukuda D (2013) Germanium recovery by catechol complexation and subsequent flow through membrane and bead-packed bed column. J Chem Technol Biotechnol 88:1468–1472
Tang S-f, Zhou C-s, Jiang X-y, Zhao C-l (2000) Extraction separation of germanium with hydroxamic acid HGS98. J Cent South Univ Technol 7:40–42
Tian R-c, Zou J, Zhou L (1984) New technology for indium, germanium and gallium recovery in an electrolytic zinc plant. In: The international conference of mineral processing and extractive. Metallurgy proceeding, Kunming, China pp 615–624
Torralvo FA, Fernández-Pereira C, MC Campanario (2011) Recovery of germanium from real fly ash leachates by ion-exchange extraction. Miner Eng 24:35–41
Tutkun O, Demircan N, Kumbasar RA (1999) Extraction of germanium from acidic leach solutions by liquid membrane technique. Clean Prod Process 1:148–153
USGS (2017): Germanium—Statistics and Information. https://www.usgs.gov/media/files/germanium-2017-pdf. Accessed 11 February 2020
USGS (2018): Germanium—Statistics and Information. https://www.usgs.gov/media/files/germanium-2018-pdf. Accessed 21 July 2021
USGS (2021) Germanium—Statistics and Information. https://pubs.usgs.gov/periodicals/mcs2021/mcs2021-germanium.pdf. Accessed 31 Jan 2021
Virolainen S, Paatero E (2013) Ion exchange recovery of germanium from sulfate solutions with N-methylglucamine functional resin. In: Conference of By-Product Metals in Non-Ferrous Metals Industry, Wroclaw, Poland
Vliegen JH, Haesebroek GG, De Schepper AJ (1990) Process for recovering germanium. WO1990013677A1, WO Patents. The World Intellectual Property Organization
Vliegen JH, Haesebroek GG, De Schepper AJ (1994) Process for recovering germanium. US5277882A, US Patents. The United States Patent and Trademark Office
Vojković V, Juranović I, Tamhina B (2001) Extraction and separation of germanium (IV) with 4-pyridone derivatives. Croat Chem Acta 74:467–477
Wang W, Wang F, Lu F (2018) Microwave alkaline roasting-water dissolving process for germanium extraction from zinc oxide dust and its analysis by response surface methodology (RSM). Metallurgical Res Technol 115:203
Wang H, Jiangshun L, Kaixi J, Dingfan Q (2006) Recovery of Ga, Ge from zinc residues by hydrometallurgical processes. In: International Symposium of Advanced Processing of Metals and Materials Proceeding, vol 7. California, USA, 413–420
Wardell MP, Davidson CF (1987) Acid leaching extraction of Ga and Ge. JOM 39:39–41
Willersinn S, Bart H-J (2016) Kinetics of Ge(IV) Extraction using a microstructured membrane contactor. Int J Chem Kinet 48:609–621
Wu X, Yuan M, Guo X, Zhang L (2019) Fast coadsorption and selective separation of gallium(III) and germanium(IV) from aqueous solutions by 3D hierarchical porous hoya-like α-FeOOH. ACS Sustain Chem Eng 7:15939–15947
Xiong J, Liang J, Fan L, Xu K, Huang Y (2012) 2012 International conference on future electrical power and energy system study on extracting of germanium with trioctylamine. Energy Procedia 17:1965–1973
Yakabe K, Minami S-i (1981) Liquid-liquid extraction of germanium(IV) with trioctylamine from aqueous oxalic acid solution. Nippon Kagaku Kaishi 1981:969–973
Yasuda S, Kawazu K (1991) Separation of germanium from ethylene glycol distillates by N-methylglucamine resin. Sep Sci Technol 26:1273–1277
Yazdi M (2008) Coal. Beheshti University Press, Tehran, Iran
Zeng L-H, Wang M-Z, Hu H, Nie B, Yu Y-Q, Wu C-Y, Wang L, Hu J-G, Xie C, Liang F-X, Luo L-B (2013) Monolayer graphene/germanium Schottky junction as high-performance self-driven infrared light photodetector. ACS Appl Mater Interfaces 5:9362–9366
Zhang T, Jiang T, Liu Z (2019) Recovery of Ge(IV) from synthetic leaching solution of secondary zinc oxide by solvent extraction using tertiary amine (N235) as extractant and trioctyl phosphate (TOP) as modifier. Miner Eng 136:155–160
Zhang T, Jiang T, Liu Z (2021) Extraction behavior of germanium from synthetic leaching solution of secondary zinc oxide in tertiary amine (N235)-trioctyl phosphate (TOP) system. Miner Eng 160:106682
Zhou T, Zhong X, Zheng L (1989) Recovering in, Ge and Ga from zinc residues. JOM 41:36–40
Zhu Y, Hu H, Guo S (2003) Title is not available (in Chinese). Chin J of Rare Metals 27:310–313
Author information
Authors and Affiliations
Contributions
M. I. and H. K. H. presented the idea for the review. H. K. H. did the literature review, data analysis, and critically revised the paper. H. K. H. finalized the paper.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kamran Haghighi, H., Irannajad, M. Roadmap for recycling of germanium from various resources: reviews on recent developments and feasibility views. Environ Sci Pollut Res 29, 48126–48151 (2022). https://doi.org/10.1007/s11356-022-20649-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-20649-5