Abstract
Our previous gene expression studies in a PCB-exposed cohort of young children in Slovakia revealed that early-life exposures to PCBs and other organochlorine compounds were associated with significant alterations across several pathogenetic pathways. The present study was undertaken to further explore the high-throughput qRT-PCR-based gene expression effects by using TaqMan low-density array (TLDA) for selected genes in a sample of 55 children from the cohort. We analyzed the transcriptional changes of 11 genes in relation to PCB and organochlorine pesticide exposure levels (including DDT, DDE, HCH, and HCB), and to BMI and ethnicity in this cohort. The results indicated an overall downregulation of expression of these genes. Maximum downregulation (in fold change) was observed in the ENTPD3 gene, and the minimum level of downregulation was in CYP2D6. As per our multinomial regression model study, downregulation of LEPR gene was significantly directly correlated with all the exposure variables. Downregulation of APC, ARNT, CYP2D6, LEPR, LRP12, and MYC genes was directly correlated with BMI (kg/m2) of the individuals. Gender-specific differences in gene expression were observed in CYP2D6 (p-value 0.0001) and LEPR (p-value 0.028), while downregulation of CYP2D6 (p-value 0.01), LEPR (p-value 0.02), LRP12 (p-value 0.04), and MYC (p-value 0.02) genes was consistently observed in Roma children compared to Caucasians. The investigation of such health disparities must be emphasized in future research, together with interventions to reduce the health consequences of PCB exposures. In this context, we emphasize the importance of biomarker-based approaches to future research on genetic susceptibility to the effects of these compounds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polychlorinated biphenyls (PCBs) are one of the most persistent environmental chemical toxicants that has been recognized by the United States Environmental Protection Agency (USEPA), The Agency for Toxic Substances and Disease Registry (ATSDR), World Health Organization (WHO), and The International Joint Commission (IJC) (USEPA, 2003; ATSDR, 2000; WHO, Food Safety; Chemical Risk in Food 2005; IJC, 2002). Due to their stability, PCBs are very persistent in the environment. Despite a ban on their production since 1979 (in the USA), body burdens of PCBs continue to accumulate in humans owing to the dumping of these compounds (Sun et al., 2007; Hsu et al., 2003). Due to its lipophilicity, the body burden of PCBs further depends on adiposity and the dietary intake of high-fat foods (Smeds and Saukko, 2001; Covaci et al., 2002; Yu et al. 2007). Thus, the legacy of environmental PCBs is truly multi-dimensional.
Due to their structural differences, the modes of action of different PCB congeners can result in different disease outcomes (Carpenter, 2006). The structure–activity relationships studies suggested that coplanar PCBs have biological activities alike dioxin (2,3,7,8-tetrachlorobibenzo-p-dioxin) through the aryl hydrocarbon receptor activity, hence a potent carcinogenic compound (Carpenter, 2006; Safe, 1994). On the other hand, the non-coplanar PCBs (e.g., 99, 138, 153, 180, and 19) show more complex patterns of toxicity, having estrogenic and neurotoxic activities (Saint-Amou et al. 2006). Both coplanar and non-coplanar PCB congeners have been detected in human tissues and in the circulation; however, the nonplanar PCBs are the most prevalent and persistent congeners in the environment (Humphrey et al., 2000).
PCBs are often categorized as organochlorines, along with organochlorine pesticides (OCPs), which in turn are the most abundant of the persistent organic pollutants in the environment. They have been extensively used in agriculture as well as in public health measure (e.g., malaria eradication) worldwide, for the over several decades, and are even used in several developing countries, whereas they are banned in most developed countries (Jayaraj et al., 2016). These pesticides are also well-known endocrine disruptors (Mnif et al., 2011). OCPs such as dichlorodiphenyltrichloroethane (DDT), hexachlorocyclohexane (HCH), and hexachlorobenzene (HCB) have been found to be carcinogenic in some studies. Based on the aforesaid information, International Agency for Research on Cancer (IARC, 2014) categorized them as “possibly carcinogenic to humans.”
PCBs have also been associated and responsible for serious chronic diseases and disorders, viz., harmful reproductive health effects (Plísková et al., 2005), neurological deficits (Park et al., 2009), endocrine effects (Rádiková et al., 2008), hearing losses (Trnovec et al. 2008), including diabetes, cardiovascular diseases, and cancers (Ghosh et al., 2014; 2015; 2018). Developmental effects from exposures to PCB congeners have also been reported (Royland et al., 2008).
In eastern Slovakia, improper disposal of PCBs over several decades caused an extended period of contamination of freshwater sediments (Kocan et al., 2001; Park et al., 2007; Wimmerová et al., 2015). Studies between 1987 and 1990 in Slovakia observed elevated concentrations of PCBs in food (Hertzman, 1995). A study from the breastfeeding mothers of Michalovce district also showed that concentrations of the PCBs in breast milk averaged from 4.0 to 4.4 mg/kg lipids (Hertzman, 1995), which greatly exceeds regulatory safety levels (< 0.01–0.04 ng/g) (Korrick and Altshul 1998). The typical PCB concentration (the sum of PCB — 28, 52, 101, 138, 153, 156 170, and 180) in human blood lipids in overall population living long term in the Michalovce District (highly contaminated) was 3.5 times higher than Stropkov District (with lower exposure only). The serological analysis revealed that PCB 153 and PCB 138 are the prevalent congeners, comparable to other studies during that time frame (Ghosh et al., 2009; Hovander et al., 2006; Petrik et al., 2006; Jursa et al. 2006). Population-based investigation in this area has also shown deleterious effects in neuro-behavioral development, and reduction in thymus size at birth (Park et al., 2008; Šovčíková et al. 2015).
To date, gene expression studies on the Slovak cohort (Ghosh et al., 2015; 2018) have showed relationships of PCB exposures with alterations in the expression levels of multiple genes in the developing disease and disorder (in pathways) that are in accord with other studies carried out by us (Ghosh et al., 2013; 2014; 2015; Mitra et al., 2012). Those prior findings suggested that certain genes were downregulated at elevated PCB exposure concentrations. However, dependency or any correlation of the gene downregulation with associated factors like DDT, DDE, HCB, or HCH co-exposures, and individual characteristics such as gender, BMI, and ethnicity were not studied in detail. The aim of the present investigation, therefore, is to address these knowledge gaps by further characterizing the expression levels of a panel of candidate genes that emerged from our prior work, in relation to PCB and OCP exposure levels and personal characteristics of children in this unique cohort.
Methods
Analysis of PCBs/POPs
Analyses of 15 PCB congeners (e.g., PCBs 28, 52, 101, 105, 138, 114, 118, 123 +149, 153, 156+171, 157, 167, 170, 180, and 189) and also p,p'-DDT, p,p'-DDE, HCB, and HCH (α, β, and γ) in the serum was done using gas chromatography (High-Resolution; 6890N; Agilent Technologies, Santa Clara, CA, USA) coupled with a Ni-63 micro-electron capture detector and a 60-m DB-5 capillary column (J&W Scientific, Folsom, CA, USA) (Kocan et al., 1994; 2001; Conka et al. 2005; Petrik et al., 2006).
Study participants
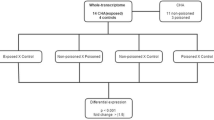
Howard University Institutional Review Board (IRB-07-GSAS-30) and Ethics Committee of the Slovak Medical University in Bratislava (Dated April 2006) approved the study. Participants included in this study were among cohort of mother–child pairs, in the study “PCBs and Early Child Development in Slovakia”, reqcruited between 2002 and 2004 (Hertz-Picciotto et al., 2003; Sonneborn et al., 2008a, b). The enrollment and description of this cohort can be found in details in Ghosh et al. (2018). We selected 71 participants (boys = 30, girls = 41) from our earlier study (Ghosh et al., 2018) and built upon their blood PCB measurements at the age of 45 months, aiming to compare and contrast the low- and high-exposure subsets. Out of the 71 participants, 55 (boys = 25, girls = 30) were included in the present study and the rest of the 16 participants were excluded due to incomplete data set. Regarding the ethnicity of the population, 13 were Roma (Gipsy) and 42 were Caucasian. The gender distribution, ethnicity, and other details of the participants are summarized in Table 1. There are no substantial variations in body weight and BMI between boys and girls and between Roma and Caucasian group in our study population.
Body mass index (BMI)
The body mass index (BMI) for all subjects was recorded at the medical clinic at the age of 45 months and was expressed in units of kg/m2 here by capturing their height and weight data during enrollment.
Sample collection and RNA preparation
Blood samples from the children at the age of 45 months, with prior parental consent, were collected into a PAXgene™ blood RNA tube (IVD; BD Biosciences) by certified phlebotomist under the direction of the medical team from Slovak Medical University (Park et al., 2007). The samples were transported to Bratislava in a cold chain prior to shipping to collaborators’ lab at USA, through special air freight carrier. The RNAs were isolated by using PAXgene Blood RNA kit (Cat # 762,164, PreAnlytiX GmbH, Germany) and TRIzol® Plus RNA Purification System (Invitrogen, California; CA), respectively (Ghosh et al., 2015; 2018). The RNA was stored at − 80 °C if not worked on immediately (within 24 h).
cDNA synthesis
High-capacity cDNA Reverse Transcription Kits (Part # 4,387,406; Applied Biosystems, CA, USA) was used for cDNA synthesis (see Ghosh et al., 2018 for detailed procedural description). The cDNAs were stored in − 15 to –25 °C, if not used immediately (within 24 h), or stored in 2 to 8 °C prior to downstream application.
High-throughput TaqMan® low density array (TLDA)
TLDA data analysis
SDS Ver. 2.4 software (ABI, CA) was used for the TLDA data analysis. Threshold cycle (Ct) data for all focused genes and control gene 18 s RNA were used to determine the ΔCt values [ΔCt = Ct (target gene) − Ct (18 s RNA)]. The ΔΔCt values were then computed by deducting the calibrator (control) from the ΔCt values for each target(s) gene. DataAssist V2.0 (ABI, CA) allowed us to visualize the expression of each gene in the corresponding individuals.
Statistical analysis
Student’s t-test was used for comparisons between two groups, including high- versus low-exposure status, and by gender and ethnicity. To determine the relationship between individual factors and gene expression, we performed linear regression analysis and checked the slope to find any observable linear association. Reported data are represented as means ± SEM. Linear regression analysis was performed using GraphPad Prism (version 8) software. The differences observed and the data with p-value < 0.05 were stated as statistically significant.
A multinomial regression model was applied (using SAS, version 9.4) on all 11 genes simultaneously, with adjustment for ethnicity, gender, BMI, and all exposure variables (PCBs, HCH, DDE, DDT, and HCB). All the variables that were not normally distributed were log-transformed prior to the analysis. Because of the significant inter-correlations between the exposure variables in the data set, we analyze one exposure variables at a time in relation to the gene expression levels and patient variables.
Results
Overall differential gene expression of 45-month old Slovak children
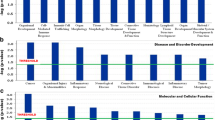
Out of the 11 candidate genes, we observed that the majority was differentially expressed (both up-/downregulated) (Fig. 1A): all ten genes were downregulated in 49 participants (89% of the population), except for the CYP2D6 gene, which was downregulated in only 63.63% (n = 35) of the total population (Fig. 1B). Maximum downregulation (fold change) was observed for ENTPD3 (− 1.21-fold change). Minimum level of expression was for CYP2D6 gene (− 0.38-fold change) (Fig. 1C).
A Quantitative real-time PCR (qRT-PCR) validation of the selected 11 genes of interest by TaqMan low-density array (TLDA) in ABI platform (7900HT Fast Real-Time PCR System) after analyzed (ΔΔCt) by SDS RQ Manager Version 1.2.1. The relative quantification (RQ) of the genes showing up-/downregulation among the subjects in a small population (n = 55) validation. The RQ is calculated in contrast to calibrator samples, i.e., the subjects with no/minimum background PCB exposures in the population. B Histogram of total percentage of population (n = 55) having downregulation of individual genes. C Histogram of average fold change (downregulation) of individual genes in overall population
A notable difference in the relative quantification of selected gene expression was observed in between boys and girls (Fig. 2), where most of the genes (except CYP2D6 and ENTPD3) were more downregulated in the boys group compared to the girls group. Out of the 11 genes, a gender-specific significant change of gene expression observed in CYP2D6 and LEPR gene with an observed p-value was 0.0001 and *0.028 respectively (Fig. 2).
PCB exposure and gene expression
Blood samples were collected from the Michalovce District, which is considered high PCB-contaminated area, where PCBs 153, 138, 180, and 170 were the most abundant PCB congeners (Ghosh et al., 2018). The average concentrations of PCB 153, 138, 180, and 170 were 185.04 ± 193.92 ng/g lipid, 123.44 ± 135.20 ng/g lipid, 132.19 ± 141.73 ng/g lipid, and 52.98 ± 57.98 ng/g lipid respectively. We did not observe any significant correlation between PCB concentration (ng/g lipid) and individual gene expression.
HCH exposure and gene expression
The mean levels of α, β, and γ–HCH in the study population were 0.91 ± 2.01, 6.34 ± 7.29, and 0.89 ± 1.67 ng/g lipid, respectively (Fig. 3A). There were no statistically significant associations of HCH with gene expression although several marginal associations (p<0.07) were observed between γ–HCH and four different gene expression (BCL2, CD3G, CYP2D6, and LRP12).
DDT, DDE, and HCB exposures in relation to gene expression
The mean levels of DDT, DDE, and HCB were 22.36 ± 81.71, 531.16 ± 385.93, and 112.30 ± 246.09 ng/g lipid, respectively (Fig. 3B). DDT, DDE, and HCB individual levels were not significantly associated with gene expression levels.
Ethnicity and relative gene expression
Gene expression levels differed by ethnicity (Fig. 4). Out of the 11 genes, an ethnicity-specific substantial difference in gene expression was observed in CYP2D6 (p-value 0.01) and LEPR (p-value 0.02), LRP12 (p-value 0.04), and MYC (p-value 0.02).
BMI and relative gene expression
Out of 11 differentially expressed genes, lower levels of expression of APC (R2 = 0.0374), ARNT (R2 = 0.0313), CYP2D6 (R2 = 0.0421), LRP12 (R2 = 0.0296), and MYC (R2 = 0.0279) were correlated with increasing BMI of the individuals (Fig. 5A–F).
Multinomial linear regression results
To assess the multiple effects of gender, ethnicity, BMI, PCBs, and other OC exposure levels on gene expression levels, we performed multinomial linear regression on the set of 11 genes. The results showed that the overall model (11 genes included) was not statistically significant (p-value 0.43) after adjusting for ethnicity, gender, BMI, and all exposure variables (sum of PCBs, α-HCH, β-HCH, γ-HCH, HCB, DDE, and DDT). We subsequently investigated the relationship of one exposure at a time with a single gene at a time, while adjusting for ethnicity, gender, and BMI. We observed a significant relationship between LEPR gene and with all the exposure variables, including sum of PCBs (p-value 0.0046), with α, β, and γ–HCH (p-value 0.0081, 0.0110, and 0.0045 respectively), DDT (p-value 0.0105), and DDE (p-value 0.0091). We also found a significant correlation between sum of PCBs and MYC (p-value 0.0449), γ–HCH and MYC (p-value 0.0596), and γ–HCH and CYP2D6 (p-value 0.0377) genes using such models.
Discussion
The current study was designed to explore the transcriptional profiling of a panel of 11 genes in PCB-exposed Slovak children using high-throughput qRT-PCR. The aim was to explore the effects of co-exposure to other persistent organochlorine exposures and the potential associations of the gene expression levels with gender, ethnicity, and BMI, which have never been reported for this cohort. The results confirm the prior findings of strong relationships between PCBs and a general pattern of downregulated gene expression. Other types of organochlorine exposures were not strongly related to gene expression levels. Increasing BMI was linearly associated with increases in expression levels of 6 of the 11 genes we profiled.
The 11 genes in this study, e.g., APC, ARNT, BCL2, CD3G, CYP2D6, ENTPD3, LEPR, LRP12, MYC, RRAD, and TRAP1 demonstrated significant expression changes, which in turn have a foremost effects in facilitating toxicities by modifying cellular and molecular events towards development of disease and disorders, i.e., cell cycle, cellular movement, cell death, cancer, metabolic disorder, neurological diseases, tumor, genetic disorder, and immunological diseases as the most common, underlying functions (Table 2), previously validated in an in vitro transcriptional profiling study (Ghosh et al., 2015) and biological pathway analysis (Ghosh et al., 2018).
As per our multinomial model results, the sum of PCBs and also α-HCH, β-HCH, γ-HCH, HCB, DDE, and DDT environmental exposures were significantly associated with the downregulation of the LEPR gene. LEPR gene was downregulated in most of the samples (94.54% of the study population), corroborating with our earlier investigation (Ghosh et al., 2013).
The LEPR gene works as a receptor for the fat cell-specific hormone leptin. It controls of fat metabolism and thereby regulates body weight, and it is also engaged in a distinctive hematopoietic pathway, important for normal lymphopoiesis (Bennett et al., 1996). The downregulation of LEPR (leptin receptor) gene disrupts the natural function of leptin. Equally, we suggest that the children previously have had the high pre- and postnatal exposure to PCBs could experience an alteration in the profile of leptin in early life. This may be linked with increased predisposition to obesity and metabolic disorders in adulthood. Earlier studies also reported that low dose exposure in young adults to p, p′-DDE (a persistent lipophilic metabolite of DDT), p, p′-DDT, and PCBs with more chlorine atoms predicted increased BMI in the future (Lee et al., 2011).
It is known that high BMI or obesity in childhood has significant impacts on both physical and psychological health (Smith et al., 2020). Overweight or obesity is now a worldwide health concern, where obese children will be most likely to remain obese rest of their lifetime. They are also more predisposed to have more non-communicable diseases like diabetes and cardiovascular diseases at much younger ages (Kelishadi and Heidari-Beni, 2019). BMI in our studied population showed a direct relationship with the transcriptional changes (downregulated) observed on selected genes (APC, ARNT, CYP2D6, LEPR, LRP12, and MYC) (Fig. 5), all of which are closely linked to the development of cancer or obesity (Ghosh et al., 2015).
The ethnicity in the studied population revealed an interesting relationship with the observed transcriptional patterns. Genetic factors play an important role that affects the risk specific diseases or sensitivity to therapeutic drugs in a conventionally defined racial group (Howard et al., 2019; Geneviève et al., 2020). Following those instances, prenatal PCB exposure in the Romani population from the same Slovak region showed an association with birth weight (Sonneborn et al., 2008a, b). The maternal PCB levels were linked with lower birth weight in Romani boys. The higher levels of PCBs in maternal blood sera may restrict the growth in boys, influenced by social factors related to ethnicity (Sonneborn et al., 2008a, b; Park et al., 2008). In our group of 45-month-old children, higher exposure to PCBs, DDE, DDT, and HCH in Romani children was associated with higher downregulation of selected genes (except CYP2D6 and ENTPD3) (Fig. 4). In view of frequent congenital malformations, consanguinity, and high incidence of genetically conditioned diseases in the Roma population (Hajioff and McKee, 2000; Bartosovic 2016; Kalaydjieva et al., 2001), our results on gene expression were not surprising. In prior research on the genetic susceptibility of the Slovak Roma population, associations were observed such as the mutation W24X in the GJB2 gene (Minárik et al. 2012), mutations in NDRG1 and HK1 genes (Gabrikova et al., 2013), occurrence of pathogenic variants in the ACADS gene (Lisyová et al., 2018), incidence of Crigler-Najjar syndrome type I (Zmetáková et al., 2007), high incidence of primary congenital glaucoma (gene symbol GLC3) (Genčík et al., 1982; Plásilová et al. 1998), higher occurrence of MCAD deficit (Bzddúch 2006), and a frequent mutation of the phenylalanine hydroxylase gene (Kalanin et al., 1994). Moreover, the Slovak inhabitants of Roma ethnicity are considered a group with a higher risk of cardiovascular disease (Hujová et al. 2010), and others have observed heterogeneity in genetic profiles of the Slovak Romany (Gypsy) sub-ethnic groups (Siváková et al., 1994; Bernasovský et al., 1994). The lack of detailed information on the health indices of minority population (Roma here), demands the need for additional research on their health and cultural concerns (Zeman et al., 2003).
ENTPD3 showed maximum downregulation (1.21-fold change) in our study that encrypts a plasma membrane-bound divalent cation-dependent E-type nucleotidase and is engaged in the control of extracellular concentrations of ATP by hydrolysis and additional nucleotides. Our result strongly corroborated and supports that the downregulation of the ENTPD3 gene during the early developmental stage may lead to functional deficits and increased risks of diabetics or even cancer in later life (Li et al., 2019), although, as per our observation, the level of gene expression did not correlate with the other important exposure conditions (e.g., DDT, DDE, HCH) (data not shown).
There is sufficient evidence that PCB exposure is associated with disease development in children, whereas a study from 2012 equally recommends that additional prenatal OC exposures including HCH, DDE, and DDT have been related to being overweight at 6.5 years of age (Valvi et al., 2012). HCH isomers are also classified as potential human carcinogens and endocrine disruptors with established teratogenic, mutagenic, and genotoxic effects. They are rapidly absorbed from the gastrointestinal tract, and they crossed the placental barrier and are also transferred into breast milk. γ-HCH has the most acute neurotoxicity followed by α-HCH, whereas less β-HCH permeates the central nervous system (Berntssen et al., 2017). In our study, β-HCH was more prevalent in the population compared to the other two isomers (α and γ), yet we observed a linear association of γ-HCH with MYC and CYP2D6 gene downregulation. Our previous study reported the prospective molecular effects of HCH exposure on a genomic level with possible molecular impairments and disease risks (Mitra et al., 2012). And finally, the effect of DDE and DDT has been extensively studied for their toxicity and carcinogenicity in animals and humans, and as endocrine disruptors (Harada et al., 2016). We also detected high amounts of DDE and DDT exposure in the study population. The level of exposure also correlated with downregulation of the LEPR gene, which is also corroborated with the earlier studies in the Slovak cohort (Ghosh et al., 2013; 2015).
In conclusion, our results suggest that environmental exposures to persistent organochlorine pollutants, especially PCBs, are associated with measurable effects on gene expression levels at the age of 45 months. In our Slovakian cohort, there are also consistent effects of male gender, Roma ethnicity, and higher BMI levels on gene expression patterns. Taken together, the results have implications for the future health of these children, for whom attentive surveillance is warranted for the future development of metabolic syndrome and other multi-systemic diseases including cancer. Efforts to mitigate such health effects of the legacy of environmental pollution must also be strengthened and extended to other regions and populations around the globe. The identification of any health disparities, i.e., the vulnerability of the Roma children in our study, must also be emphasized in such ongoing and future research, together with interventions to reduce the health consequences of such disparities. Finally, we emphasize the importance of biomarker-based approaches to future research on genetic susceptibility.
Data availability
Not applicable.
References
ATSDR (2000) Toxicological profile for polychlorinated biphenyls (PCBs). US Public Health Service, Agency for Toxic Substances and Disease Registry; Atlanta, GA:. Available from: https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=142&tid=26. (Accessed 30 October 2021).
Bartosovic I (2016) Some aspects of health status of the Gypsy population in Slovakia. Bratisl Lek Listy 117(1):26–30. https://doi.org/10.4149/bll_2016_006
Bennett BD, Solar GP, Yuan JQ, Mathias J, Thomas GR, Matthews W (1996) A role for leptin and its cognate receptor in hematopoiesis. Curr Biol 6:1170–1180. https://doi.org/10.1016/s0960-9822(02)70684-2
Bernasovský I, Halko N, Biros I, Siváková D, Jurícková J (1994) Some genetic markers in Valachian (Olachian) Gypsies in Slovakia. Gene Geogr 8(2):99–107
Berntssen MHG, Maage A, Lundebye A-K (2017) Chemical contamination of finfish with organic pollutants and metals. In: Schrenk, D., Cartus, A., (Ed.) Chemical contaminants and residues in food. Duxford (United Kingdom): Woodhead Publishing. 517–550. https://doi.org/10.1016/j.chemosphere.2009.12.021
Bzddúch V (2006) Deficit MCAD - eastejsí výskyt v rómskej populácii [MCAD deficit–its higher occurrence in the Roma population]. Cas Lek Cesk 145(11):886
Carpenter DO (2006) Polychlorinated biphenyls (PCBs): routes of exposure and effects on human health. Rev on Environ Health 21:1–23. https://doi.org/10.1515/reveh.2006.21.1.1
Conka K, Drobná B, Kocan A, Petrík J (2005) Simple solid-phase extraction method for determination of polychlorinated biphenyls and selected organochlorine pesticides in human serum. J Chromatogr A 1084(1–2):33–38. https://doi.org/10.1016/j.chroma.2004.11.029
Covaci A, Jorens P, Jacquemyn Y, Schepens P (2002) Distribution of PCBs and organochlorine pesticides in umbilical cord and maternal serum. Sci Total Environ 298:45–53. https://doi.org/10.1016/s0048-9697(02)00167-5
Gabrikova D, Mistrik M, Bernasovska J, Bozikova A, Behulova R, Tothova I, Macekova S (2013) Founder mutations in NDRG1 and HK1 genes are common causes of inherited neuropathies among Roma/Gypsies in Slovakia. J Appl Genet 54(4):455–460. https://doi.org/10.1007/s13353-013-0168-7
Gencik A, Gencikova A, Ferák V (1982) Population genetical aspects of primary congenital glaucoma I Incidence, prevalence, gene frequency, and age of onset. Hum Genet 61(3):193–7. https://doi.org/10.1007/BF00296440
Geneviève LD, Martani A, Shaw D, Elger BS, Wangmo T (2020) Structural racism in precision medicine: leaving no one behind. BMC Med Ethics 21(1):17. https://doi.org/10.1186/s12910-020-0457-8
Ghosh S, Dutta S, Zang S, Trnovec T, Palkovicova L, Sovcikova E, Ghimbovschi S, Hoffman E (2009) PCB exposure in vitro (PBMC): differential gene expression, pathway analysis for possible mode(s) of actions, and disease development in comparison with PCB-exposed Slovak population, ISEE 21st Annual Conference. Dublin, Ireland, Epidemiology 20(6):S130. https://doi.org/10.1097/01.ede.0000362447.94784.b4
Ghosh S, Loffredo CA, Mitra PS, Trnovec T, PalkovicovaMurinova L, Sovcikova E, Hoffman EP, Makambi KH, Dutta SK (2018) PCB exposure and potential future cancer incidence in Slovak children: an assessment from molecular finger printing by Ingenuity Pathway Analysis (IPA®) derived from experimental and epidemiological investigations. Environ. Sci. Pollut. Res. Int. 25(17):16493–16507. https://doi.org/10.1007/s11356-017-0149-1
Ghosh S, Mitra PS, Loffredo CA, Trnovec T, Murinova L, Sovcikova E, Ghimbovschi S, Zang S, Hoffman EP, Dutta SK (2015) Transcriptional profiling and biological pathway analysis of human equivalence PCB exposure in vitro: indicator of disease and disorder development in humans. Environ Res 138:202–216. https://doi.org/10.1016/j.envres.2014.12.031
Ghosh S, Murinova L, Trnovec T, Loffredo CA, Washington K, Mitra PS, Dutta SK (2014) Biomarkers linking PCB exposure and obesity. Curr Pharm Biotechnol 15(11):1058–1068. https://doi.org/10.2174/1389201015666141122203509
Ghosh S, Trnovec T, Palkovicova L, Hoffman EP, Washington K, Dutta SK (2013) Status of LEPR gene in PCB-exposed population: a quick look. Int J Hum Genet 13(1):27–32. https://doi.org/10.1080/09723757.2013.11886193
Hajioff S, McKee M (2000) The health of the Roma people: a review of the published literature. J Epidemiol Community Health 54(11):864–869. https://doi.org/10.1136/jech.54.11.864
Harada T, Takeda M, Kojima S, Tomiyama N (2016) Toxicity and carcinogenicity of dichlorodiphenyltrichloroethane (DDT). Toxicol Res 32(1):21–33. https://doi.org/10.5487/TR.2016.32.1.021
Hertzman C (1995) Environment and health in Eastern Europe. A report for the Environmental Action Programme for Central and Eastern Europe. World Bank. https://doi.org/10.1596/0-8213-3173-6
Hertz-Picciotto I, Trnovec T, Kočan A, Charles MJ, Čižnar P, Langer P, Sovčikova E, James R (2003) PCBs and early childhood development in Slovakia: study design and background. Fresen Environ Bull 12(2):208–214
Hovander L, Linderholm L, Athanasiadou M, Athanassiadis I, Bignert A, Fängström B, Kocan A, Petrik J, Trnovec T, Bergman A (2006) Levels of PCBs and their metabolites in the serum of residents of a highly contaminated area in eastern Slovakia. Environ Sci Technol 40(12):3696–3703. https://doi.org/10.1021/es0525657
Howard VJ, Madsen TE, Kleindorfer DO, Judd SE, Rhodes JD, Soliman EZ, Kissela BM, Safford MM, Moy CS, McClure LA, Howard G, Cushman M (2019) Sex and race differences in the association of incident ischemic stroke with risk factors. JAMA Neurol 76(2):179–186. https://doi.org/10.1001/jamaneurol.2018.3862
Hsu YK, Holsen TM, Hopke PK (2003) Locating and quantifying PCB sources in Chicago: receptor modeling and field sampling. Environ Sci Technol 37:681–690. https://doi.org/10.1021/es025531x
Hujová Z, Alberty R, Ahlers I, Ahlersová E, Paulíková E, Desatniková J, Gábor D, Hrubá F (2010) Cardiovascular risk predictors in central Slovakian Roma children and adolescents: regional differences. Cent Eur J Public Health 18(3):139–144. https://doi.org/10.21101/cejph.a3563
Humphrey HE, Gardiner JC, Pandya JR, Sweeney AM, Gasior DM, McCaffrey RJ, Schantz SL (2000) PCB congener profile in the serum of humans consuming great lakes fish. Environ Health Perspec 108:167–172. https://doi.org/10.1289/ehp.00108167
IARC (2014) Polychlorinated biphenyls and polybrominated biphenyls. IARC Monogr Eval Carcinog Risk Chem Hum 107
IJC (2002) 11th Biennial Report -Great Lakes Water Quality, IJC 11th Biennial Report -Great Lakes Water Quality. Available from: https://www.ijc.org/sites/default/files/saint2012ar.pdf. (Accessed 28 October 2021)
Jayaraj R, Megha P, Sreedev P (2016) Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip Toxicol 9(3–4):90–100. https://doi.org/10.1515/intox-2016-0012
Jursa S, Chovancová J, Petrík J, Loksa J (2006) Dioxin-like and non-dioxin-like PCBs in human serum of Slovak population. Chemosphere 64(4):686–691. https://doi.org/10.1016/j.chemosphere.2005.10.048
Kalanin J, Takarada Y, Kagawa S, Yamashita K, Ohtsuka N, Matsuoka A (1994) Gypsy phenylketonuria: a point mutation of the phenylalanine hydroxylase gene in Gypsy families from Slovakia. Am J Med Genet 49(2):235–239. https://doi.org/10.1002/ajmg.1320490215
Kalaydjieva L, Gresham D, Calafell F (2001) Genetic studies of the Roma (Gypsies): a review. BMC Med Genet 2:5. https://doi.org/10.1186/1471-2350-2-5 (Epub 2001 Apr 2)
Kelishadi R, Heidari-Beni M (2019) Prevention and control of childhood obesity: the backbone in prevention of non communicable disease. Adv Exp Med Biol 1121:61–66. https://doi.org/10.1007/978-3-030-10616-4_7
Kocan A, Petrík J, Drobná B, Chovancová J (1994) Levels of PCBs and some organochlorine pesticides in the human population of selected areas of the Slovak Republic. I Blood Chemosphere 29(9–11):2315–2325. https://doi.org/10.1016/0045-6535(94)90400-6
Kocan A, Petrik J, Jursa S, Chovancova J, Drobna B (2001) Environmental contamination with polychlorinated biphenyls in the area of their former manufacture in Slovakia. Chemosphere 43(4–7):595–600. https://doi.org/10.1016/s0045-6535(00)00411-2
Korrick SA, Altshul L (1998) High breast milk levels of polychlorinated biphenyls (PCBs) among four women living adjacent to a PCB-contaminated waste site. Environ Health Perspect 106(8):513–518. https://doi.org/10.1289/ehp.98106513
Lee DH, Lind PM, Jacobs DR Jr, Salihovic S, van Bavel B, Lind L (2011) Polychlorinated biphenyls and organochlorine pesticides in plasma predict development of type 2 diabetes in the elderly: the prospective investigation of the vasculature in Uppsala Seniors (PIVUS) study. Diabetes Care. 34:1778–1784. https://doi.org/10.2337/dc10-2116
Li M, Qi Y, Chen M, Wang Z, Zeng D, Xiao Y, Li S, Lin H, Wei X, Zhang G (2019) GATA binding protein 3 boosts extracellular ATP hydrolysis and inhibits metastasis of breast cancer by up-regulating ectonucleoside triphosphate diphosphohydrolase 3. Int J Biol Sci 15(12):2522–2537. https://doi.org/10.7150/ijbs.35563
Lisyová J, Chandoga J, Jungová P, Repiský M, Knapková M, Machková M, Dluholucký S, Behúlová D, Šaligová J, Potočňáková Ľ, Lysinová M, Böhmer D (2018) An unusually high frequency of SCAD deficiency caused by two pathogenic variants in the ACADS gene and its relationship to the ethnic structure in Slovakia. BMC Med Genet 19(1):64. https://doi.org/10.1186/s12881-018-0566-0
Minárik G, Tretinárová D, Szemes T, Kádasi L (2012) Prevalence of DFNB1 mutations in Slovak patients with non-syndromic hearing loss. Int J Pediatr Otorhinolaryngol 76(3):400–403. https://doi.org/10.1016/j.ijporl.2011.12.020
Mitra PS, Ghosh S, Zang S, Sonneborn D, Hertz-Picciotto I, Trnovec T, Palkovicova L, Sovcikova E, Ghimbovschi S, Hoffman EP, Dutta SK (2012) Analysis of the toxicogenomic effects of exposure to persistent organic pollutants (POPs) in Slovakian girls: correlations between gene expression and disease risk. Environ Int 39(1):188–199. https://doi.org/10.1016/j.envint.2011.09.003
Mnif W, Hassine AI, Bouaziz A, Bartegi A, Thomas O, Roig B (2011) Effect of endocrine disruptor pesticides: a review. Int J Environ Res Public Health 8(6):2265–2303. https://doi.org/10.3390/ijerph8062265
Park HY, Hertz-Picciotto I, Petrik J, Palkovicova L, Kocan A, Trnovec T (2008) Prenatal PCB exposure and thymus size at birth in neonates in eastern Slovakia. Environ Health Perspect 116:104–109. https://doi.org/10.1289/ehp.9769
Park JS, Linderholm L, Charles MJ, Athanasiadou M, Petrik J, Kocan A, Drobna B, Trnovec T, Bergman A, Hertz-Picciotto I (2007) Polychlorinated biphenyls and their hydroxylated metabolites (OH-PCBS) in pregnant women from eastern Slovakia. Environ Health Perspect 115(1):20–27. https://doi.org/10.1289/ehp.8913
Park JS, Petreas M, Cohn BA, Cirillo PM, Factor-Litvak P (2009) Hydroxylated PCB metabolites (OH-PCBs) in archived serum from 1950–60s California mothers: a pilot study. Environ Int 35(6):937–942. https://doi.org/10.1016/j.envint.2009.04.002
Petrik J, Drobna B, Pavuk M, Jursa S, Wimmerova S, Chovancova J (2006) Serum PCBs and organochlorine pesticides in Slovakia: age, gender, and residence as determinants of organochlorine concentrations. Chemosphere 65(3):410–418. https://doi.org/10.1016/j.chemosphere.2006.02.002
Plásilová M, Feráková E, Kádasi L, Poláková H, Gerinec A, Ott J, Ferák V (1998) Linkage of autosomal recessive primary congenital glaucoma to the GLC3A locus in Roms (Gypsies) from Slovakia. Hum Hered 48(1):30–33. https://doi.org/10.1159/000022778
Plísková M, Vondrácek J, Canton RF, Nera J, Kocan A, Petrík J, Trnovec T, Sanderson T, van den Berg M, Machala M (2005) Impact of polychlorinated biphenyls contamination on estrogenic activity in human male serum. Environ Health Perspect 113(10):1277–1284. https://doi.org/10.1289/ehp.7745
Rádiková Z, Tajtáková M, Kocan A, Trnovec T, Seböková E, Klimes I, Langer P (2008) Possible effects of environmental nitrates and toxic organochlorines on human thyroid in highly polluted areas in Slovakia. Thyroid 18(3):353–362. https://doi.org/10.1089/thy.2007.0182
Royland JE, Wu J, Zawia NH, Kodavanti PR (2008) Gene expression profiles in the cerebellum and hippocampus following exposure to a neurotoxicant, Aroclor 1254: developmental effects. Toxicol Appl Pharmacol 231(2):165–178. https://doi.org/10.1016/j.taap.2008.04.022
Safe SH (1994) Polychlorinated biphenyls (PCBs): environmental impact, biochemical and toxic responses, and implications for risk assessment. Crit Rev Toxicol 24(2):87–149. https://doi.org/10.3109/10408449409049308
Saint-Amou D, Roy MS, Bastien C, Ayotte P, Dewailly E, Després C, Gingras S, Muckle G (2006) Alterations of visual evoked potentials in preschool Inuit children exposed to methylmercury and polychlorinated biphenyls from a marine diet. Neurotoxicology 27(4):567–578. https://doi.org/10.1016/j.neuro.2006.02.008
Siváková D, Sieglová Z, Lubyová B, Nováková J (1994) A genetic profile of Romany (Gypsy) subethnic group from a single region in Slovakia. Gene Geogr 8(2):109–116
Smeds A, Saukko P (2001) Identification and quantification of polychlorinated biphenyls and some endocrine disrupting pesticides in human adipose tissue from Finland. Chemosphere 44:1463–1471. https://doi.org/10.1016/s0045-6535(00)00313-1
Smith JD, Fu E, Kobayashi MA (2020) Prevention and Management of Childhood Obesity and Its Psychological and Health Comorbidities. Annu Rev Clin Psychol 16(1):351–378. https://doi.org/10.1146/annurev-clinpsy-100219-060201
Sonneborn D, Park HY, Babinska K, Palkovicova L, Trnovec T, Kocan A, Nguyen DV, Hertz-Picciotto I (2008a) Serum PCB concentrations in relation to locally produced food items in eastern Slovakia. J Expo Sci Environ Epidemiol 18(6):581–587. https://doi.org/10.1038/jes.2008.1
Sonneborn D, Park HY, Petrik J, Kocan A, Palkovicova L, Trnovec T, Nguyen D, Hertz-Picciotto I (2008b) Prenatal polychlorinated biphenyl exposures in eastern Slovakia modify effects of social factors on birth weight. Paediatr Perinat Epidemiol 22(3):202–213. https://doi.org/10.1111/j.1365-3016.2008.00929.x
Šovčíková E, Wimmerová S, Strémy M, Kotianová J, Loffredo CA, Murínová ĽP, Chovancová J, Čonka K, Lancz K, Trnovec T (2015) Simple reaction time in 8–9-year old children environmentally exposed to PCBs. Neurotoxicology 51:138–144. https://doi.org/10.1016/j.neuro.2015.10.005
Sun P, Basu I, Blanchard P, Brice KA, Hites RA (2007) Temporal and spatial trends of atmospheric polychlorinated biphenyl concentrations near the Great Lakes. Environ Sci Technol 41:1131–1136. https://doi.org/10.1021/es061116j
Trnovec T, Sovčíková E, Hust’ák M, Wimmerová S, Kočan A, Jurečková D, Langer P, Palkovičová L, Drobná B (2008) Exposure to polychlorinated biphenyls and hearing impairment in children. Environ. Toxicol. Pharmacol. 25(2):183–7. https://doi.org/10.1016/j.etap.2007.10.030
USEPA. Second National Report on human exposure to environmental chemicals. Baltimore, MD: U.S. Environmental Protection Agency; 2003. Available from: https://cfpub.epa.gov/si/si_public_record_Report.cfm?dirEntryID=58071. (Accessed 17 November 2021).
Valvi D, Mendez MA, Martinez D, Grimalt JO, Torrent M, Sunyer J, Vrijheid M (2012) Prenatal concentrations of polychlorinated biphenyls, DDE, and DDT and overweight in children: a prospective birth cohort study. Environ Health Perspect 120(3):451–457. https://doi.org/10.1289/ehp.1103862
WHO, Food Safety; Chemical Risk in Food (2005) Available from: https://www.who.int/news-room/fact-sheets/detail/food-safety. (Accessed 11 November 2021)
Wimmerová S, Watson A, Drobná B, Šovčíková E, Weber R, Lancz K, Patayová H, Richterová D, Koštiaková V, Jurečková D, Závacký P, Strémy M, Jusko TA, Palkovičová Murínová Ľ, Hertz-Picciotto I, Trnovec T (2015) The spatial distribution of human exposure to PCBs around a former production site in Slovakia. Environ Sci Pollut Res Int 22(19):14405–14415. https://doi.org/10.1007/s11356-015-5047-9
Yu Z, Palkovicova L, Drobna B, Petrik J, Kocan A, Trnovec T, Hertz-Picciotto I (2007) Comparison of organochlorine compound concentrations in colostrum and mature milk. Chemosphere 66(6):1012–1018. https://doi.org/10.1016/j.chemosphere.2011.09.013
Zeman CL, Depken DE, Senchina DS (2003) Roma health issues: a review of the literature and discussion. Ethn Health 8(3):223–249. https://doi.org/10.1080/1355785032000136434
Zmetáková I, Ferák V, Minárik G, Ficek A, Poláková H, Feráková E, Kádasi L (2007) Identification of the deletions in the UGT1A1 gene of the patients with Crigler-Najjar syndrome type I from Slovakia. Gen Physiol Biophys 26(4):306–310
Funding
This study was supported by a U54 (MD007597-31–5959) grant (PI/PD: Southerland, Lead PI: Ghosh) from NIMHD (NIH), and a P20 grant (CA242617-03–7897) (PI: Ghosh) from the NCI (NIH). This study also received prior support from the following: grant number 1UO1ES016127‐01 from the National Institute of Environmental Health Sciences (NIEHS/NIH); the European Commission-funded 7FP project OBELIX (No. 227391); the Ministry of Health, Slovak Republic, through funded projects 2007/07-SZU-03, 2012/41-SZU-5, and 2012/47-SZU-11; Slovak Research and Development Agency funding through projects APVV-0571–12 and APVV-0444–11; and the project “Centre of Excellence of Environmental Health” (ITMS No. 26240120033) based on the supporting Operational Research and Development Program financed from the European Regional Development Fund. This work also received support from the NCI (NIH) grants R01-CA96525 and R03 TW007152 (co-PI: Trnovec).
Author information
Authors and Affiliations
Contributions
TM, SG, and CL together conceptualized the work and manuscript. TM—writing–original draft preparation. LM and TT completed the epidemiological, medical, and background data collection; ZN and TN performed the laboratory work. KC and BD—PCB analysis; CL—statistical analysis. TT, CL, LM, and SG—writing, review, and editing.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Howard University Institutional Review Board (IRB-07-GSAS-30) and by the Ethics Committee of the Slovak Medical University in Bratislava (dated April 2006).
Consent to participate
All participates agreed to participate in this study and signed the informed consents.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mondal, T., Loffredo, C.A., Trnovec, T. et al. Gene expression signatures in PCB-exposed Slovak children in relation to their environmental exposures and socio-physical characteristics. Environ Sci Pollut Res 29, 60531–60541 (2022). https://doi.org/10.1007/s11356-022-20018-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-20018-2