Abstract
Human remains have been interred in burial grounds since historic times. Although the re-use of graveyards differs from one country, region or time period to another, over time, graveyard soil may become contaminated or enriched with heavy metal elements. This paper presents heavy metal element soil analysis from two UK church graveyard study sites with contrasting necrosols, but similar burial densities and known burial ages dating back to the sixteenth century and some possibly older than 1,000 years. Portable X-ray fluorescence element laboratory-based analyses were undertaken on surface and near-surface soil pellets. Results show elevated levels of Fe, Pb, Mn, Cr, Cu, Zn and Ca in both necrosols when compared with background values. Element concentration anomalies remained consistently higher than background samples down to 2 m, but reduced with distance away from church buildings. Element concentration anomalies are higher in the clay-rich necrosol than in sandy necrosol. Study result implications suggest that long-used necrosols are likely to be more contaminated with heavy metal elements than similar soil outside graveyards with implications for burial grounds management, adjacent populations and where burial grounds have been deconsecrated and turned to residential dwellings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Burial grounds are unique both in their natural environment, including soil type, parent material, vegetation, topography and climate, and in their anthropogenic burial numbers, styles, depths, body distributions and above-ground placement of memorials, buildings and installation of pathways and roads to access the site (Hansen et al. 2014). The mechanical disturbance via re-excavation and re-infilling of burial sites, and varying aboveground vegetation types, as well as the presence of human remains, makes graveyard soils unique and have their own necrosol soil type category (Amuno and Amuno 2014; Asare et al. 2020). Necrosols, despite increasing numbers of cemeteries and burial sites both in rural and in urban environments, are poorly understood, especially regarding potential contamination and ecological risks (Jonker and Oliver 2012). This is largely due to the complex biological and chemical processes occurring in these soils, resulting in both spatial and temporal heterogeneity of necrosols (Amuno and Amuno 2014).
Reuse of graveyard and cemetery sites for burying human remains has been happening for at least 10,000 years since Early Mesolithic times (Schulting et al. 2019). The practice of reusing existing graveyards differs spatially by country and region and relating to timing of clearing old graves before new ones are emplaced. For example, the USA generally leaves human remains untouched in situ in perpetuity, whereas in the UK it is common to have a 100-year period, by which time any direct relatives should have died before an existing graveyard can be reused (Mytum 2000), and in Germany remains can be moved when only buried for 25 years and a fresh grave is emplaced (Fiedler et al. 2009).
Soil from graveyard sites differ from the natural soil profile largely through disturbance and due to the nature of the material buried. Previous research has likened graveyards to landfills (Fiedler et al. 2012), with elevated levels of organic matter (Kim et al. 2008), embalming fluids (Chiappelli and Chiappelli 2008; Uslu et al. 2009) and creosote from coffins (Mininni et al. 2007), as well as materials from the bodies themselves including Hg and Au from teeth fillings (Fiedler et al. 2012). In a few cases, cemetery materials carried in soil water have also been found to have contaminated local groundwater supplies with pathogens, viruses and heavy metals (Konefes and McGee 2000; Matias et al. 2004; Kim et al. 2008).
Non-invasive geophysical studies in such burial grounds indicate elevated conductivity levels in grave soils (Hansen et al. 2014), with individual grave geophysical anomalies decreasing with increasing burial age, compared to background values. However, soil texture and moisture content have been shown to be major variables with sandy soils causing leaching of grave contents well beyond the grave-cut, whereas clay-rich soils tend to retain these fluids within the grave-cut itself (Pringle et al. 2012, 2016; Dick et al. 2017).
Archaeological studies have shown ancient burial ground soils to have elevated levels of heavy metal elements such as Fe, Pb, Mn and Cu (Amuno and Amuno 2014, Jonker and Oliver 2012), as well as other elements such as P and N (Bethell and Carver 1987; Asare et al. 2020), with human exposure to such toxic metals causing kidney damage (Khan et al. 2011) and links to Parkinson’s and Alzheimer’s disease (Mohod and Dhote 2013). This may have important health implications for residents in the surrounding areas as well as potential risks to the local environment via leaching into surrounding soils and groundwater (Jonker and Oliver 2012). However, there has been, to date, limited research on the soil contamination potential of cemeteries and graveyards.
X-ray fluorescence spectrometry (XRF) is an analytical technique for determining total element concentrations in a wide variety of materials and is used in environmental applications. Traditional, laboratory-based, spectrometry including inductively coupled plasma mass spectrometry, atomic emission spectroscopy and optical emission spectrometry, among others, are widely used to determine trace and heavy metal elemental concentrations in soils (Schneider et al. 2016; Messager et al. 2021). These methods have high analytical precision, but involve higher costs per sample, and lengthier sample preparation often involving heavy use of acids for digestion, data processing and analytical time.
Portable XRF (pXRF) field surveys have been shown to be effective for rapid evaluation of heavy metal soil contamination (Radu and Diamond 2009; Brent et al. 2016; Rouillon et al. 2017; Liang et al. 2018), biogeochemical mapping over mine tailings (Rincheval et al. 2019), archaeological object studies (Kasztovsky et al. 2018; Michalowski et al. 2020), marine microplastics (Turner 2017), species profiling (Nganvongpanit et al. 2016) and even lead levels in living human bones (Zhang et al. 2020). This highlights that pXRF is a powerful technique for rapid elemental analysis of soils and can be used both in situ and within the laboratory (Frahm and Doonan 2013; Goff et al. 2019). There is currently no universally agreed protocol for pXRF sample preparation related to soil analysis (Goff et al. 2019). For soil investigations, fresh soils can be challenging due to their highly variable water contents across different land uses, soil types and habitats, which can be somewhat problematic when conducting physical measurements for site comparisons, for example, electrical resistivity surveys (Jervis and Pringle 2014). This holds true for pXRF analysis, with the additional complication that the attenuation of X-rays by water is a function of the energy used to characterise the elements of interest. Low-energy X-rays are more strongly attenuated than high-energy ones; thus, for pXRF analysis, elements with lower atomic masses are more strongly impacted. Studies have shown that for every 1% increase in soil water content, there is a 1.15–1.75% decrease in reported elemental concentration for Mn through to As, whilst elements lighter than Mn are even more greatly attenuated (Parsons et al. 2013; Imanishi et al. 2010). This means that sample water content must be measured and corrected for, as light element values determined in situ will likely not be directly comparable to results of a traditional laboratory analysis (Padilla et al. 2019). Goff et al. (2019) compared different sample preparation techniques for pXRF analysis and identified that a pressed powder pellet provides best results and avoids fluorescence attenuation from field moist soil conditions.
Here we utilise the pressed pellet method to evaluate the potential heavy metal contamination of long-used (500 + years) church necrosol graveyards with different soil textures. Study objectives were therefore to (1) use pressed powder soil pellets from topsoils from 2 contrasting soils from long-established church graveyards and analyse for heavy metals using pXRF, (2) assess the respective known burial population and areal extent to quantify the respective graveyard burial histories and (3) identify potential soil and water quality implications for those living adjacent to burial grounds or living on ex-burial grounds.
Materials and methods
Graveyard site background
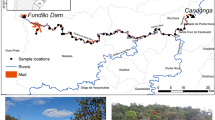
Two Church of England graveyards were selected for this study, study site 1 at St. John’s Church in Keele Staffordshire, UK (Fig. 1a), and study site 2 at St. Michael and All Angels’ Church in Stockton, Norfolk, UK (Fig. 1b). These were selected as they have burial records from over 500 years ago to the present day, contrasting soil textures and bedrock types (Dick et al. 2017), different rainfall levels and geographic settings.
Study site 1 is located in the rural Keele Village in Staffordshire, situated ~ 200 m above sea level and with an average rainfall of 806 mm/year. The site was a Knights Templar church built around 1160 CE, before being taken over by the Knights Hospitaller in 1324 CE. A new church was built by the local Sneyd family in the sixteenth century, before the present sandstone church was built in 1868–1870 CE (Pevsner 1974). A desktop study, confirmed by soil auger (Fig. 1a), showed there to be a sandy loam soil overlying the Upper Carboniferous Butterton Sandstone Member of the Halesowen Formation bedrock found at ~ 2.5 m below ground level (bgl).
Study site 2 is located in the rural Stockton village in south Norfolk, situated ~ 35 m above sea level, with an average rainfall of 620 mm/year. The church of St. Michael and All Angels is present on the study site, with a Saxo-Norman-style round tower, probably dating from the thirteenth to the fourteenth century, and with later medieval flintwork additions (Knott 2005). A desktop study, confirmed by soil auger (Fig. 1b), showed there to be clay-rich soil, derived from a glacial diamicton, overlying clays and sands of the Pleistocene Beccles Formation found at ~ 2.5 m bgl.
Known burial records
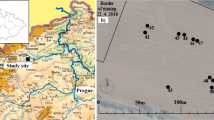
For site 1 at St. Johns, graveyard burial records showed there were 5,735 individuals from 1585 to 1970 CE and 1990 to 2018 CE (see Fig. 2a and Supplemental Data). The burial data gap was due a records office fire. Average yearly burials were 13/year, with a typical increase after the Industrial Revolution (the church being situated on the edge of the industrialised area of Stoke-on-Trent), before declining in the twentieth century.
For site 2 at St. Michaels, graveyard burial records there were 669 individuals from 1561–2018 CE (see Fig. 2b and Supplemental Data). Average yearly burials were 1/year, with a typical increase and decrease in burial rate with the Industrial Revolution and the twentieth century, respectively, as observed in case study 1.
When burial numbers were corrected for the areal extent of the graveyards (10,800 m2 for study site 1 at St. John’s and 2,330 m2 for study site 2 at St. Michaels), burial densities of ~ 2.2 individuals/m2 at study site 1 and ~ 3 individuals/m2 at study site 2 were determined.
Both graveyards had mostly marked earth-cut graves with headstones, with burial ages that range from the eighteenth century to the present day (Fig. 2). At study site 1, there were more multiple familial burials in the same grave which results in more variable coffin burial depths. A general review by Hart and Casper (2004) found average depths (bgl) of 1.4 m for one coffin, 1.83 m for two coffins and 2.7 m for four coffins for familial burials.
Soil sampling and analysis
Research on optimal sampling methodologies has suggested at least five samples should be acquired in different locations on a site to gain representative results (Pye et al. 2005, 2007; McKinley and Ruffell 2007). Field reconnaissance in these two study sites determined the soil sampling areas that could be used, for example, avoiding areas of dense vegetation/trees and aboveground memorials, tarmac paths, etc., which prohibited sampling. A random number generator was then used to locate to avoid potential sampling bias within 5 m × 5 m grids at study site 1 and study site 2 respectively. Two sample arable field locations in arable fields in the same soil type were also ~ 100 m away from each graveyard (Fig. 2) which were used to act as a control to quantify local soil background element values.
pXRF surface soil sampling
Overlying vegetation was firstly removed to expose the soil at 33 and 31 sampling locations at study sites 1 and 2, respectively, which was then checked for pebbles or vegetation which would contaminate the measurement (Fig S1).
Approximately 250 g of surface soil from the top 5 cm was collected from each sampling location at both study sites, bagged, labelled and stored at 4 °C. A subset of samples was also taken for routine soil characterisation analysis, initially including determination of average electrical conductivity, soil pH and water contents following standard soil characterisation methodologies.
To create the pressed powder pellet, surface soil samples were oven-dried for 24 h at 105 °C before being hand-ground using a pestle and mortar to pass through a 63-μm sieve. The sample was then mixed with 1.5 mL of polyvinylpyrrolidone-methylcellulose binding agent and mechanically pressed under 10-tn pressure (Fig S1) into a homogenous flat-cylinder pellet, before being oven-dried for a further 24 h at 105 °C to remove any soil water influence on signal strength (Kalnicky and Singhvi 2001). Each pellet (Fig S1) was then analysed using the NITON XL3t 900 pXRF Analyzer in its laboratory holder, using a 5-min measurement time, chosen as a compromise between analytical time and instrumental precision (see Pringle et al. 2022). The instrument employs four sub-filters (Main, Low, High, Light), each targeting a specific range of elements. Each of these filters was allotted an equal proportion of the overall analytical time. Analytical precision, expressed as two standard deviations of repeat analyses of the NIST2709 international reference material, was 1% for Fe and Ti, 2% for Ca and < 5% for Mn, Zn and Cr. Precision for Pb was 14%, and 28% for Cu and As, due to low concentrations. All analytes with the exception of As and Cr were quantified using a user calibration, calibrating the instrument using a range of international reference materials (AGV-1, RGM-1, QLO-1, NIM-S and DR-N). For these analytes, accuracy (expressed as percentage deviation from the recognised values of the NIST2709 international reference material) was quantified at 1% for Fe and Ti; < 6% for Mn, Zn and Ca; and < 15% for Cu. By contrast, analysis of As and Cr was found to yield more accurate results using the in-built, factory-standard Niton calibration of the instrument, and yielded accuracies of 32% and 18%, respectively.
Soil depth profile
To determine element variation at depth, 0.75 m (n = 3) soil cores were taken using a handheld slimline hand auger at 32 and 31 sampling locations at study site 1 and study site 2 graveyards respectively. Cores were split into 0.25-m intervals (0–0.25 cm, 0.26–0.50 cm and 0.51–0.75 cm respectively) (Fig S1). The resulting soil samples were then labelled and stored in polyethylene bags at 4 °C until soil pellets from each of the listed soil depth intervals were generated using the procedure as already detailed above and then each pXRF analysed in the laboratory for a 5-min measurement time as previously described.
Single site deep soil core
A grave digger was hand-digging a new grave in study site 1 whilst soil sampling was being conducted, so opportunistically the research team were able to collect deeper subsoil samples. Here, ~ 250 g of soil samples was collected every 0.25-m down to 2-m depth on the south end of the empty grave (Fig S1). The resulting soil samples were labelled and stored in polyethylene bags at 4 °C until soil pellets were generated and pXRF analysed in the laboratory for a 5-min measurement time procedure as already detailed.
Results
Basic soil characterisation
The samples had routine soil characterisation analysis, including determination of electrical conductivity, pH and soil moisture content following standard methodologies. Study site 1 electrical conductivity (EC) of soils recorded an average of 47 μS/cm (28 SD), with study site 2 recording an average EC of 99 μS/cm (49 SD). Study site 1 pH of soils displayed an average of 6.3 (0.8 SD), with study site 2 recording an average of 7.8 (0.8 SD). Study site 1 soil moisture content ranged from 8 to 27% (average 14.1%), with study site 2 soil moisture content ranging from 13 to 27% (average 21%).
pXRF surface soil sampling
Our results show clear differences in element concentrations in the soil samples measured, when compared to the background control samples, within each study site graveyard and between the two graveyards summarised in Tables 1 and 2.
At study site 1 with the sand-rich soil, surface soil pXRF measurements showed Pb element concentrations varying from 69 mg/kg up to a maximum of 742 mg/kg with an average of 188 mg/kg (Table 1), with relatively higher concentrations adjacent to the church itself (Fig. 3a). Control soil Pb element concentration average was only 30 mg/kg. Mn also had high concentrations, varying from 77 mg/kg up to a maximum of 2,409 mg/kg with an average of 1177 mg/kg (Table 1), with background Mn soil concentration average of 798 mg/kg. As had relatively low concentrations, varying from 4 up to 18 mg/kg with an average of 8 mg/kg, the same concentration as the control soil samples (Table 1). Cu had an average of 29 mg/kg with control soil sample being below detection levels (Table 1). Ca had an average of 19,317 mg/kg with control soil sample averages being 1738 mg/kg.
At study site 2 with the clay-rich soil, surface soil pXRF measurements showed very high Pb concentrations, varying from 38 mg/kg up to a maximum of 2,310 mg/kg with an average of 317 mg/kg (Table 1), with higher concentrations adjacent to the church (Fig. 3b). Control soil Pb element concentration averages was only 28 mg/kg. Zinc had also high concentrations, varying from 64 up to 6,528 mg/kg, with an average of 532 mg/kg (Table 2). The Zn distribution across the graveyard was more varied when compared to the lead distributions, with background Zn soil average of 67 mg/kg. Arsenic had similar concentrations as background control samples, varying from 3 up to 21 mg/kg with an average of 8 mg/kg. Cu had an average of 26 mg/kg with control soil sample being 13 mg/kg (Table 1). Ca had an average of 37,709 mg/kg with control soil sample averages being 4492 mg/kg.
Comparing element concentrations between graveyards, Pb concentrations were higher in the clay soil of study site 2 compared to the sandy soil of study site 1 which has been reported elsewhere (see Yuan et al. 2014).
As soil sample locations were geospatially referenced, elements could be compared directly with the analysis of distance from the respective churches at each study site (Fig. 4). Pb, Cu and Zn elements showed decreasing concentration trends with increasing distance from the church buildings but these were not statistically significant. Ca levels were recorded at very high concentrations adjacent to site 2 church building (> 100,000 mg/kg) when compared to ~ 5,000 mg/kg average control soil values. This was expected due to the lime plaster building construction that was present here but still useful to evidence.
Variations down a soil depth profile
At the sand-rich soil study site 1, Pb concentrations were consistently high, averaging 287 mg/kg at 0–0.25 m, 434 mg/kg at 0.26–0.5 m and 381 mg/kg at 0.51–0.75 m bgl (Fig. 5), which contrasts with 30-mg/kg control levels. As concentrations were low averaging ~ 10 mg/kg that was similar to background values (Table 3). Cu concentrations were ~ 64 mg/kg through the soil depth profile with control soil values being below detectable levels. Fe concentrations increased with soil depth in both the graveyard and control values and Ca decreased with soil depth.
At the clay-rich soil study site 2, Zn and Pb element concentrations increase with increasing soil depth bgl, with Pb in particular increasing, averaging from 39 mg/kg at 0–0.25 m, 142 mg/kg at 0.26–0.5 m to 608 mg/kg at 0.51–0.75 m bgl (Fig. 5), which contrasts with the 31-mg/kg control sample concentrations. As concentrations were low and similar to the control average of 4–7 mg/kg (Table 4). Cu concentrations were ~ 12–21 mg/kg through the soil depth profile with control soil values being below detectable levels. Fe and Ca concentrations decreased with soil depth and Fe values were about twice as high as control soil Fe values.
Single-site deep soil core
Here at the sandy soil study site 1, Fe, Zn, Mn and Cr element concentrations generally increased with soil depth bgl with Cu constant and As and Ca decreasing with soil depth (Fig. 6). Ca concentrations reduced with depth to 600 mg/kg at 2 m bgl, slightly higher than the national average of 400 mg/kg for sandy rural soils as given by Ross et al. (2007). These results (Table 5) reinforce the soil auger profile results although note this was only one empty grave sample at one location within the study site 1 graveyard.
Discussion
Here, we aimed to evaluate the potential heavy metal contamination of long-used (500 + years) church necrosol graveyards with different soil types. The graveyard datasets acquired from surface soil (0–0.05 m), shallow soil depths (> 0.75 m) and single deep soil (> 2 m) (at study site 1 only) all showed heavy metal concentrations were not only higher than background control soil samples taken ~ 100 m away from both graveyards (Tables 1–5), but were also higher than average concentrations observed in the soils of England and Wales (Ross et al. 2007). Measured Pb and As element concentrations were well above the threshold level (75 mg/kg) identified as potentially resulting in ecotoxicological effects (deVries et al. 2007). In many of the graveyard soil sampling points examined, Pb concentrations were also above the predicted ‘no effect’ concentrations of 166 mg/kg and 212 mg/kg reported by Smolders et al. (2009) and the European Chemicals Agency (ECA-Ecotoxicological Summary for lead), respectively. Our paper shows heavy metals were present in both study site soils at much higher concentrations than those found in other studies, with Neckel et al. (2016), for example, only recording Pb values up to 127 mg/kg (Table 6). Fiedler et al. (2012) graveyard study evidenced comparable Pb levels from this study to an uncovered coffin (Table 6). It should be noted that As concentrations were generally low at both study sites and did not exceed either control values or WHO soil standards of ~ 10 mg/kg, even though it is not very mobile in alkaline soil (Fiedler et al. 2012).
There were also large variations of measured heavy metal element concentrations within each graveyard, with the highest concentrations being found generally in soil samples taken adjacent to church buildings. This would suggest building materials would be a major source of heavy metals found in the adjacent soils such as Pb flashings, particularly as element concentrations decreased with increasing spatial distance from the churches. Other elements also showed this trend such as Ca, which may suggest a higher burial density nearest the church and corresponding release of elements from coffins as Fiedler et al. (2012) measured from graveyard coffins in Germany (Table 6). Generally, soil samples showed the same trend of decreasing element concentrations with increasing soil depth, except for Pb at study site 1 with sandy soil which had consistently high lead values down to 0.75 m. As given in the introduction, the coffins themselves are often a source of metal contamination so burial concentration should be a factor when looking at characterising necrosols.
Comparing the two graveyard study sites, although the number of known burials and graveyard areal extent was different, the actual burial density was similar (~ 2.5 m2), so element concentration differences may be due to the different soil type, with clay-rich soils having greater reactive surface areas which can bind metals and other mineral and organic substances in the environment (Weil and Brady 2017). Surface soil (and down to 0.25 m bgl) heavy metal concentrations were generally higher in the clay soil at study site 2, when compared to the sandy soil at study site 1, suggesting elements are less mobile in the low porosity/permeability clay soils. In contrast, higher element concentration values occur in deeper soils in the sandy study site 1 but unfortunately deeper samples than 0.75 m bgl were not collected from the clay soil at study site 2 so it cannot be stated definitively that higher element levels are not present at depth in this graveyard.
This study has important implications for managing both historical and contemporary burial grounds, in relation to re-use and potential environmental and ecological contamination impacts from burial sites. Depending upon the soil type, as evidenced here, mobile heavy metals may leach away from the graveyard area itself and potentially to nearby surface and groundwater supplies if the geological conditions are suitable for this, as detailed by Oliveira et al. (2012) and Matias et al. (2004) evidenced from a Portuguese graveyard study and nearby water borehole results. Finally, a number of closed churches, graveyards and cemeteries are being deconsecrated and turned into residential dwellings with bodies being commonly left rather than being exhumed and reburied in nearby burial grounds. Soil analysis for heavy metal concentrations in such grounds would be highly recommended especially if people living in these areas wanted to grow edible produce which could bioaccumulate these heavy metals in their tissues. Further research is needed on these converted site to assess this important environmental and human health contamination risk.
Future work
Whilst one empty grave at the study site 1 was able to be sampled, it would obviously be advantageous to sample grave soil deeper and ideally adjacent to coffins themselves as per Fiedler et al. (2012) adipocere study. Other burial ground types, for example green or ‘natural’ burials, are becoming increasingly popular globally, with 270 UK sites being built between 1993 and 2015 alone (Yarwood et al. 2015). These generally have lower burial densities, when compared to cemetery/graveyard burial grounds, biodegradable receptacles (e.g. shrouds, cardboard or wicker-based), but involve more shallow or even vertical burials (Kim et al. 2008). The study of element mobility in graveyard soils should also be undertaken to determine which necrosols are likely to be more contaminated. These factors would suggest early decomposition stages releasing more fluids, including embalming fluids, into the surrounding soil, when compared to more traditional burials, with accompanying increased surrounding soil contamination, but little research has been undertaken on this to-date.
Conclusions
This paper provides two case studies of long-used (500 + years) burial grounds, UK church graveyards in this case, whose necrosols are contaminated by heavy metals. Here we used pXRF to determine the spatial extent (distance and depth) of heavy metal contaminations, and highlight its use for rapid data collection across different environmental samples. In particular, we identified Pb concentrations were well in excess of current environmental guidelines, although these concentrations are not uniformly distributed, both in extent across the graveyards and in depth below ground level. The highest levels of contamination are in the top 0.25 m and adjacent to church structures, potentially due to high burial concentrations and/or due to relict church materials being incorporated into the soils. This will be important for burial ground management, those living adjacent to burial grounds, potential surface/groundwater contamination and where burial grounds have been deconsecrated and turned into residential dwellings.
This paper is limited by only studying two UK graveyards, albeit long-used with different soil types, and by the numbers of soil samples collected, analysed and measured. However, the implications for other church graveyards to be similarly contaminated is clear. More accurate analytical equipment should be used to refine these initial results and obtain absolute element measurements to further validate the use of pXRF in determining heavy metal contamination as this could help speed up and reduce costs for soil testing in potentially contaminated sites. Here, we highlight graveyards are potential repositories for heavy metals but could also be possible stores for other emerging environmental contaminations too, such as pharmaceuticals which may persist in graveyard soils after decomposition. Further research is needed to explore other graveyards, cemeteries, green burials and other burial grounds with different burial ages, in other soil types, as well as collecting soil within and adjacent to graves in order to assess environmental contamination risks as well as future environmental sustainability.
Data availability
pXRF data and burial records for the two respective case study sites are available on Keele’s eRepository, the DOI link of which is: http://doi.org/10.21252/sjkk-w810
References
Aitkenhead-Peterson JA, Owings CS, Alexander MB, Larison N, Bytheway JA (2012) Mapping the lateral extent of human cadaver decomposition with soil chemistry. Forensic Sci Int 216:127–134
Amuno SA, Amuno MM (2014) Geochemical assessment of two excavated mass graves in Rwanda: A pilot study. Soil Sed Contam 23:144–165
Asare MO, Šmejda L, Horák J, Holodňák P, Černý M, Pavlů V, Hejcmanet M (2020) Human burials can affect soil elemental composition for millennia – analysis of necrosols from the Corded Ware Culture graveyard in the Czech Republic. Archaeol Anthrop Sci 12:255
Barbieri CB, Sarkis JES, Martinelli LA, Bordon ICA, Mitteregger H Jr, Antônio Hortellani M (2014) Forensic evaluation of metals (Cr, Cu, Pb, Zn), isotopes (δ13C and δ15N), and C: N ratios in freshwater sediment. Environ Forensics 15:134–146
Bethell PH, Carver MOH (1987) Detection and enhancement of decayed inhumations at Sutton Hoo. In: Boddington A, Garland AN, Janaway RC (eds) Death, decay and reconstruction: approaches to archaeology and forensic science. Manchester University Press, Manchester, pp 10–21
Bocharnikova E, Matichenkov V, Jiang J, Yuejin C (2017) Si-based technologies for reduction of the pollutant leaching from landfills and mine tails. J Environ Tech 38:1606–1609
Brent RN, Wines H, Luther J, Irving N, Collins J, Drake BL (2017) Validation of handheld X-ray fluorescence for in situ measurement of mercury in soils. J Environ Chem Eng 5(1):768–776. https://doi.org/10.1016/j.jece.2016.12.056
Carter DO, Tibbett M (2009) Cadaver decomposition and soil: processes. In: Tibbett M, Carter DO (eds) Soil Analysis in Forensic Taphonomy: Chemical and Biological Effects of Burial Human Remains. CRC Press, Boca Raton, pp 29–52
Chiappelli J, Chiappelli T (2008) Drinking grandma: the problem of embalming. Journal of Environment Health 71:24–28
Dick HC, Pringle JK, Wisniewski KD, van der Putten R, Evans G, Goodwin J, Cassella JP et al (2017) Determining geophysical responses from burials in graveyards and cemeteries. Geophysics 82:B245-255
Environmental Agency (2006) The determination of metals in solid environmental samples: methods for examination of waters and associated materials. Available: www.gov.uk/government/uploads/system/uploads/attachment_data/file/316812/Book_204.pdf Last accessed 6th May 2018.
Fiedler S, Illich B, Berger J, Graw M (2009) The effectiveness of ground-penetrating radar surveys in the location of unmarked burial sites in modern cemeteries. J Appl Geophys 68:380–385
Fiedler S, Breuer J, Pusch CM, Holley S, Wahl J, Ingwersen J, Graw M (2012) Graveyards – special landfills. Sci Total Environ 419:90–97
Frahm E, Doonan RCP (2013) The technological versus methodological revolution of portable XRF in archaeology. J Archaeol Sci 40(2):1425–1434. https://doi.org/10.1016/j.jas.2012.10.013
Gamble SC, Campos LC, Morgan R (2017) Detection of trace peroxide explosives in environmental samples using solid phase extraction and liquid chromatography mass spectrometry. Environ Forensics 18:50–61
Goff K, Schaetzl RJ, Chakraborty S, Weindorf DC, Kasmerchak C, Bettis EA III (2019) Impact of sample preparation methods for characterizing the geochemistry of soils and sediments by portable X-ray flourescence. Soil Sci Soc Am J 84:131–143
Hansen JD, Pringle JK, Goodwin J (2014) GPR and bulk ground resistivity surveys in graveyards: locating unmarked burials in contrasting soil types. Forensic Sci Int 237:e14–e29
Hart A, Casper S (2004) Potential groundwater pollutants from cemeteries. Environmental Agency Report, ISBN-1844323471, pp. 35.
Hu Y, Liu X, Bai J, Shih K, Zeng EY, Cheng H (2013) Assessing heavy metal pollution in the surfave soils of a region that had undergone three decades in intense industrializa and urbanization. Environ Sci Pollut Res Int 20(9):6150–6159
Imanishi Y, Bando A, Komatani S, Wada S-I, Tsuji K (2010) Experimental parameters for XRF of soils. Adv. X ray Anal. 53:248–255.
Iwegbue CA (2013) Chemical fractionation and mobility of heavy metals in soils in the vicinity of asphalt plants in Delta State, Nigeria. Environ Forensics 14:248–259
Jervis JR, Pringle JK (2014) A study of the affect of seasonal climatic factors on the electrical resistivity response of three experimental graves. J Appl Geophys 108:53–60
Jonker C, Oliver J (2012) Mineral contamination from cemetery soils: case study of Zandfontein cemetery, South Africa. Int J Environ Res Pub He 9:511–520
Kasztovszky Z, MarótiB Harsányi I, Párkányi D, Szilágyi V (2018) A comparative study of PGAA and portable XRF used for non-destructive provenancing archaeological obsidian. Quat Int 468179–189. https://doi.org/10.1016/j.quaint.2017.08.004
Khalilova H, Mammadov V (2016) Assessing the anthropogenic impact on heavy metal pollution of soils and sediments in urban areas of Azerbaijan’s oil industrial region. Pol J Environ Stud 25(1):159–166
Khan SA, Din ZU, Zubair I, Zubair A (2011) Levels of selected heavy metals in drinking water of Peshawar City. Int J Sci Nat 2:648–652
Kim KH, Hall ML, Hart A, Pollard SJT (2008) A survey of green burial sites in England and Wales and an assessment of the feasibility of a groundwater vulnerability tool. Environ Technol 29:1–12
Knott S (2005) St Michael, Stockton. Available online: http://www.norfolkchurches.co.uk/stockton/stockton.htm Last accessed 14th March 2019.
Konefes JL, McGee MK (2000) Old cemeteries, arsenic and health safety Cultural resources management. National Park Service 9:15–18
Kulkarni P, Chellam S, Flanagan JB, Jayanty RKM (2007) Microwave digestion – ICP-MS elemental analysis in ambient airborne fine particulate matter: Rare earth elements and validation using a filter borne fine particle certified reference material. Anal Chim Acta 599:170–176
Liang J-H, Liu P-P, Chen Z, Sun G-X, Li H (2018) Rapid evaluation of arsenic contamination in paddy soils using field portable x-ray fluorescence spectrometry. J Environ Sci 64:345–351
Loska K, Wiechula D, Korus I (2004) Metal contamination of farming soils affected by industry. Environ Int 30:159–165
Luoma SN, Rainbow PS (2008) Metal contamination in aquatic environments: science and lateral management. Cambridge University Press, New York
Matias MJ, Marques da Silva M, Goncalves L, Peralta C, Grangeia C, Martinho E (2004) An investigation into the use of geophysical methods in the study of aquifer contamination by graveyards. Near Surf Geophys 2:131–136
McKinley J, Ruffell A (2007) Contemporaneous spatial sampling at scenes of crime: advantages and disadvantages. Forensic Sci Int 172:196–202
Messager ML, Davies IP, Levin PC (2021) Development and validation of in-situ and laboratory X-ray fluorescence (XRF) spectroscopy methods for moss biomonitoring of metal pollution. MethodsX 8:101319. https://doi.org/10.1016/j.mex.2021.101319
Michalowski A, Niedzielski P, Teska M, Jakubowski K, Zolkiewski M (2020) Archaeometrical studies of prehistoric pottery using portable ED-XRF. Measurement 159:107758. https://doi.org/10.1016/j.measurement.2020.107758
Mininni G, Sbrilli A, Braguglia CM, Guerriero E, Marani D, Rotatori M (2007) Dioxin, furans and polycyclic aromatic hydrocarbons emissions from a hospital and cemetery waste incinerator. Atmos Environ 41:8527–8536
Mohod CV, Dhote J (2013) Review of heavy metals in drinking water and their effect on human health. Int J Innov Res Sci Eng Tech 2:2992–2996
Müller G (1969) Index of geo-accumulation in sediments of Rhine River. GeoJournal 2:108–118
Mytum H (2000) Recording and analysing graveyards, Council for British Archaeology. Practical Handbooks in Archaeology 1:45–46
Neckel A, Goncalves AC Jr, Ribeiro LA, de Ameida Silva CCO, Cardoso GT (2016) Cemeteries heavy metals concentration analysis of soils and the contamination risk for the surrounding resident population. Int J Eng Res Appl 6:30–35
Nganvongpanit K, Buddhachet K, Klinhom S, Kaewmong P, Thitaram C, Mahakkanukrauh P (2016) Determining comparative elemental profile using handheld XRF in humans, elephants, dogs and dolphins: preliminary study for species identification. Forensic Sci Int 263:101–106
Oliveira B, Quinteiro P, Caetano C, Nadais H, Arroja L, da Silva AF, Senos Matias M (2012) Burial grounds impact on groundwater and public health: an overview. Water Environ J 27:99–106
Padilla JT, Hormes J, Selim HM (2019) Use of pXRF: effect of thickness and antecedent moisture of soils on measured concentration of trace elements. Geoderma 337:143–149
Parsons C, Grabulosa EM, Pili E, Floor GH, Roman-Ross G, Charlet L (2013) Quantification of trace arsenic in soils by field-portable X-ray fluorescence spectrometry: Considerations for sample preparation and measurement conditions. J Hazard Mater 2621213–1222. https://doi.org/10.1016/j.jhazmat.2012.07.001
Pevsner N (1974) Architectural guides to buildings of England: Staffordshire. Yale University Press, London
Pringle JK, Jervis JR, Hansen JD, Cassidy NJ, Jones GM, Cassella JP (2012) Geophysical monitoring of simulated clandestine graves using electrical and ground penetrating radar methods: 0–3 years. J Forensic Sci 57:1467–1486
Pringle JK, Jervis JR, Roberts D, Dick HC, Wisniewski KD, Cassidy NJ, Cassella JP (2016) Geophysical monitoring of simulated clandestine graves using electrical and ground penetrating radar methods: 4–6 years. J Forensic Sci 61:309–321
Pringle JK, Jeffery AJ, Ruffell A, Stimpson IG, Pirrie D, Bergslien E, Madden C, Oliver I, Wisniewski KD, Cassella JP, Lamont N, Gormley S, Partridge J (2022) The use of pXRF as a forensic geoscience non-destructive trace evidence tool for environmental and criminal investigations. Forensic Sci Int 332:111175. https://doi.org/10.1016/j.forsciint.2022.111175
Pye K, Blott S, Croft DJ, Carter JF (2005) Forensic comparison of soil samples: assessment of small-scale spatial variability in elemental composition, carbon and nitrogen isotope ratios, colour, and particle size distribution. J Forensic Sci 163:59–80
Radu T, Diamond D (2009) Comparison of soil pollution concentrations determined using AAS and pXRF techniques. J Hazard Mat 171:1168–1171
Rincheval M, Cohen DR, Hemmings FA (2019) Biogeochemical mapping of metal contamination from mine tailings using field-portable XRF. Sci Total Environ 662:404–413
Ross M, Wood MD, Copplestone D, Warriner P, Crook P (2007) UK soil and herbage pollutant survey report 7: environmental concentrations of heavy metals in UK soil and herbage. Available online at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/291161/scho0607bmta-e-e.pdf last Accessed: 27th September 2021.
Rouillon M, Taylor MP, Dong C (2017) Reducing risk and increasing confidence of decision making at a lower cost: In-situ pXRF assessment of metal-contaminated sites. Environ Pollut 229780–789. https://doi.org/10.1016/j.envpol.2017.06.020
Schneider AR, Cances B, Breton C, Ponthieu M, Morvan X, Conreux A, Marin B (2016) Comparison of field portable XRF and aqua regia / ICPAES soil analysis and evaluation of soil moisture influence on FPXRF results. J Soils Sediments 16:438–448
Schulting RJ, Booth T, Brace S, Diekmann Y, Thomas M, Meiklejohn C, Babb J, Budd C, Charlton S, Van der Plicht H, Mullan G, Wilson L (2019) Avelines’s Hole: an unexpected twist in the tale. Proceedings of the University of Bristol Spelaeological Society 28:9–63
Smolders E, Oorts K, Sprang PV, Schoeters I, Janssen CR, McGrath SP, McLaughlin MJ (2009) Toxicity of trace metals in soil as affected by soil type and aging after contamination: using calibrated bioavailability models to set ecological soil standards. Environ Toxi Chem 28:1633–1642
Sutherland RA (2000) Bed sediment-associated trace metals in an urban stream, Oahu Hawaii. Environ Geol 39:611–627
Thompson D (2014) Rapid production of cyclonic spray chambers for inductively coupled plasma applications using low cost 3D printer technology. J Anal Atom Spectrom 29:2262
Turner A (2017) In situ elemental characterisation of marine microplastics by portable XRF. Mar Pollut Bull 124:286–291
Uslu A, Baris E, Erdogan E (2009) Ecological concerns over cemeteries. Afr J Agric Res 4(13):1505–1511
de Vries W, Lofts S, Tipping E, Meili M, Groenenberg JE, Schütze G (2007) Impact of soil properties on critical concentrations of cadmium, lead, copper, zinc, and mercury in soil and soil solution in view of ecotoxicological effects. in: Rev Environ Contam T 191, Springer, New York.
Weil RR, Brady NC (2017) The nature and properties of soils, 15th edition, Pearson Education, ISBN 978–0–13–325448–8.
Yarwood R, Sidaway JD, Kelly C, Stillwell S (2015) Sustainable deathstyles? The geography of green burials in Britain. The Geographic Journal 181:172–184
Young KE, Evans CA, Hodges KV, Bleacher JE (2016) A review of the handheld XRF spectrometer as a tool for field geologic investigations on Earth and in planetary surface exploration. Appl Geochem 72:77–87
Yuan Z, Cheng Q, Xia Q, Yao L, Chen Z, Zuo R, Xu D (2014) Spatial patterns of geochemical elements measured on rock surfaces by portable X-ray fluorescence: application to hand specimens and rock outcrops. Geoch: Expl Env, Anal 14:265–276
Zhang X, Specht AJ, Wells E, Weisskopf MG, Weuve J, Nie LH (2020) Evaluation of a portable XRF device for in vivo quantification of lead in bone among a US populations. Sci Total Environ 753:142351. https://doi.org/10.1016/j.scitotenv.2020.142351
Acknowledgements
The authors thank the Reverends P. Jones of St. John’s Church, Keele, Staffordshire, UK, and J. Oddy-Bates of St. Michael and All Angels, Stockton, Norfolk, UK; and their congregations are thanked for respective site access and for allowing this project to be conducted. Keele University undergraduate and postgraduate taught students are thanked for data collection, soil preparation and analysis assistance.
Funding
The Nuffield Foundation are acknowledged for providing financial assistance for summer research placements for L. Hall, T. Cooke, T. Coombes, L. Barber, N. Lawrence, M. Chan, N. Ekechukwu, R. Perager, H-C Chan and S. Lal. H. D. acknowledges the Nigerian Tertiary Education Fund for the financial support.
Author information
Authors and Affiliations
Contributions
C. M. undertook the desk study, collected field and laboratory data and performed the literature review, with M.E. collecting field/lab data and analysis. J. P. designed the study, wrote the initial draft and coordinated the authors. A. J. assisted with data analysis and calibrations and co-wrote the paper. K. W. assisted with field data collection and figure generation and co-wrote the paper. V. J., I. O., H. G., I. G. S. and J.G. co-wrote the paper.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This project has passed the Keele University's research ethics panel review.
Consent to publish
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Kitae Baek.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Madden, C., Pringle, J.K., Jeffery, A.J. et al. Portable X-ray fluorescence (pXRF) analysis of heavy metal contamination in church graveyards with contrasting soil types. Environ Sci Pollut Res 29, 55278–55292 (2022). https://doi.org/10.1007/s11356-022-19676-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-19676-z