Abstract
This review aims to understand the impacts of plasticizers on the thyroid system of animals and humans. The thyroid gland is one of the earliest endocrine glands that appear during embryogenesis. The thyroid gland synthesizes thyroid hormones (TH), triiodothyronine (T3), and thyroxine (T4) that are important in the regulation of body homeostasis. TH plays critical roles in regulating different physiological functions, including metabolism, cell growth, circadian rhythm, and nervous system development. Alteration in thyroid function can lead to different medical problems. In recent years, thyroid-related medical problems have increased and this could be due to rising environmental pollutants. Plasticizers are one such group of a pollutant that impacts thyroid function. Plasticizers are man-made chemicals used in a wide range of products, such as children’s toys, food packaging items, building materials, medical devices, cosmetics, and ink. The increased use of plasticizers has resulted in their detection in the environment, animals, and humans. Studies indicated that plasticizers could alter thyroid function in both animals and humans at different levels. Several studies demonstrated a positive and/or negative correlation between plasticizers and serum T4 and T3 levels. Plasticizers could also change the expression of various TH-related genes and proteins, including thyroid-stimulating hormone (TSH), thyrotropin-releasing hormone (TRH), and transporters. Histological analyses demonstrated thyroid follicular cell hypertrophy and hyperplasia in response to several plasticizers. In conclusion, plasticizers could disrupt TH homeostasis and the mechanisms of toxicity could be diverse.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Different types of compounds have been used as a plasticizer. In the 1850s, castor oil was used as a plasticizer, but in the 1870s, camphor became the popular plasticizer. However, camphor’s high volatility and unpleasant odor motivated the search for an alternative. In 1920, phthalate was discovered, and in 1933, the introduction of di-2-(ethylhexyl) phthalate (DEHP) opened the market for softer polyvinyl chloride materials (Graham 1973). At present, there are more than 100 different plasticizers produced worldwide, but only half of them are commercially important (Godwin 2017). Their common classifications are based on their chemical composition. The main types of plasticizers are phthalate esters, dibasic acid ester, epoxy plasticizers, glycol derivatives, benzoates, trimellitates, phosphate ester, and citrate (Bui et al. 2016).
Plasticizers are used in numerous products, such as children’s toys, household items, medical devices, clothing, food packaging materials, construction materials, and cosmetics (Kavlock et al. 2002). Plasticizers are added in plastic materials to increase their plasticity, flexibility, and durability, while in cosmetics to hold color and scent. They are also used as solvents in paints, glue, and insect repellent. Plasticizers are not covalently bound to the products; hence, they can easily leach out from the material into the environment (Bauer and Herrmann 1997). Due to this, plasticizers are accumulating in the ecosystems and detected in aquatic systems, drinking water, air dust, soils, and sewage treatment plants at substantial concentrations. Plasticizers are also found in food items, including milk products, meat, fish, and other high-fat-containing food (Barnabe et al. 2008; Bilal et al. 2019; Horn et al. 2004; Larsson et al. 2017). Hence, there are concerns over their use in everyday items.
Humans and animals are exposed to plasticizers through inhalation, oral ingestion, and skin contact. Plasticizers have negative effects on different physiological functions, including the immune system, metabolism, reproduction, brain function and cell differentiation (Barakat et al. 2017; Dobrzynska, 2016; Heudorf et al. 2007). The global market of plasticizers is expected to reach 10.5 billion tonnes worldwide by 2026 (Caresana, 2019). Hence, it is essential to determine their toxicities and monitor their environmental levels to avoid long-term impacts on humans and animals. Plasticizers are also known to influence the thyroid hormone (TH) system. The alteration of thyroid homeostasis by plasticizers could be through binding to TH receptors or carrier proteins, including transthyretin (TTR), and altering the transport protein, including sodium iodide symporter (NIS) (Boas et al. 2012; Zoeller, 2005). This review aims at understanding the impacts of plasticizers on the TH system in animals and humans.
Commonly used plasticizers and their toxicity
Di-(2-ethylhexyl) phthalate (DEHP)
DEHP is commonly used as a plasticizer in a wide range of plastic materials, such as medical devices, pharmaceuticals, cosmetics, personal care products, consumer products, building, and furniture materials (Kastner et al. 2012; Magdouli et al. 2013). DEHP is the most widely used phthalate, and as a result, it is abundant in the environment (Gimeno et al. 2014). The safe limit of DEHP concentration in drinking water has been determined as 6 and 8 µg/L by the US Environmental Protection Agency (U.S. EPA, 2012) and the World Health Organization (WHO, 2011), respectively. According to US EPA Chemical Data Reporting (CDR) database, the production volume of DEHP ranged between 45,359 and 113,398 tonnes in 2015 (U.S. EPA, 2020a).
Among the phthalates, DEHP contamination is highest in the aquatic environments with concentrations up to 97.8 µg/L in surface waters, 182 µg/L in sewage effluents, 154 mg/kg (dry weight) in sewage sludge, and 8.44 mg/kg in sediments (Fromme et al. 2002; Magdouli et al. 2013; Wang et al. 2008; Zeng et al. 2008). DEHP metabolite, mono-(2-ethylhexyl) phthalate (MEHP), has been reported to be up to 1.3 µg/L in river (Suzuki et al. 2001) and 57.2 ng/L and 0.84 ng/g dry weight in seawater and sediments, respectively (Blair et al. 2009). DEHP has also been detected in poultry, cooking oils, and cream-based dairy products with concentrations above 300 µg/kg (Serrano et al. 2014).
Several studies have demonstrated that DEHP is toxic and has potential carcinogenic properties, and due to this, it is listed as a category 1B chemical (Bui et al. 2016). In rats, DEHP negatively impacted reproduction, as it decreased sperm number and delayed vaginal opening (Andrade et al. 2006; Grande et al. 2006; Lee et al. 2009). DEHP is also known to increase lipid accumulation in different model systems by altering key genes in the fatty acid metabolism (Chen et al. 2016; Zhang et al. 2017).
Di-butyl phthalate (DBP)
DBP is widely used in several products, including medical devices, cosmetics, paper coating, plastic piping, and food wrap. It is indicated that humans are exposed to DBP through water, air, and food items (Pan et al. 2006; Wei et al. 2011). DBP has been reported at concentrations ranging from 0.6 µg/L to 1472 mg/L in the environment (Fatoki and Noma, 2002; Fatoki and Ogunfowokan 1993; He et al. 2019; Paluselli et al. 2018; Tyler et al. 1998). The limit concentration of DBP in drinking water has been determined to be 3 µg/L (Sha et al. 2007). According to the European Food Safety Authority (EFSA), the tolerable daily intake of DBP has been suggested to be 10 µg/kg body weight (bw)/day, but it has been shown that the daily intake in China is 12.2 µg/kg bw/day (Guo et al. 2011).
Several studies have linked DBP with adverse health effects. A study demonstrated that exposure to DBP could induce histopathological changes in the spleen and cause oxidative stress leading to inflammation and apoptosis (Wang et al. 2020). DBP was shown to decrease body weight and increase maternal death, malformations in fetuses, and post-implantation loss during pregnancy in rats (Ema et al. 1993). Studies on zebrafish showed that DBP has teratogenic effects (Ortiz-Zarragoitia et al. 2006), alters plasma sex hormone levels (Chen et al. 2019), and results in abnormal heart rate and pericardial edema (Mu et al. 2020).
Diethyl phthalate (DEP)
DEP is extensively used as a softener, coating material, and cosmetic additive in different products, such as hair sprays, perfumes, nail polish solvents, after-shave lotions, detergents, and shampoos (Kamrin and Mayor 1991; Kang et al. 2010). DEP is easily leached into the environment and can contaminate biological systems during DEP-containing product’s synthesis and use (Kang et al. 2010). In a survey performed in 1999–2000 on 2540 samples collected from participants of the National Health and Nutrition Examination Survey, DEP and its metabolite monoethyl phthalate (MEP) were detected in more than 75% of the urine samples (Silva et al. 2004). DEP has been detected at concentration of 0.6 µg/L in river water samples (Fatoki and Vernon 1990). The maximum detected levels of DEP were 632.2 mg/kg in household dust, 5481 ng/m3 in the indoor air of apartments, and 1263 ng/m3 in the indoor air of kindergartens (Fromme et al. 2004). The minimal risk level of DEP was determined to be 7 mg/kg bw/day in the USA (Duty et al. 2005). DEP was detected in human semen with a mean level of 0.47 μg/L (Zhang et al. 2006).
In vitro treatment of human high-density lipoproteins with DEP resulted in oxidation, aggregation, and degradation of lipoproteins and promoted foam cell formation (Kim et al. 2015). In the same study, exposure of zebrafish embryos to DEP showed embryo death, developmental speed decrease, and skeletal development impairment (Kim et al. 2015). Exposure to DEP was shown to alter liver and serum enzyme levels in young male Sprague–Dawley rats (Sonde et al. 2000), while other studies did not find any impact of DEP in rats (Foster et al. 1980) and mice (Lamb et al. 1987).
Alternative plasticizers and their toxicity
The reported toxicity of DEHP and other phthalates has resulted in the search for alternatives with low toxicity. Henceforth, alternative plasticizers have been introduced without sufficient risk assessment, and their use is overtaking the traditional plasticizers (Bui et al. 2016). In many products, including phones and computers, the banned plasticizers are excluded, but it is not specified which alternative plasticizers are being used (Pecht et al. 2018). In the European market, the use of alternative plasticizers has increased steadily. Of the alternative plasticizers, diisononyl phthalate (DINP) and diisononyl cyclohexane-1,2-dicarboxylate (DINCH) are the most widely used worldwide.
Diisononyl phthalate (DINP)
DINP is a high molecular weight alternative plasticizer with a backbone of 7–13 carbon atoms. This increases its permanency and durability (Revathy and Chitra 2018). DINP is used in different materials, such as household products, food wrapping, cosmetics, toys, paints, lubricants, and adhesives (Agency 2010). Since it is not chemically bound to polymers, DINP can enter the environment via leaching, migration, evaporation, and/or abrasion (Forner-Piquer et al. 2018). DINP has been detected in the floor and multi-surface dusts with 100% detection rates and maximum concentrations of 2100 and 15,500 µg/g dust, respectively (Ait Bamai et al. 2014). DINP metabolites have also been detected in human urine samples (Koch et al. 2013). As reported by the CDR database, the production volume of DINP was between 100 and 500 million pounds in the USA by 2015 (U.S. EPA, 2020b).
Several mammalian studies have found negative impacts of DINP on the reproductive system, including induction of nipple retention, alteration of sperm number and motility, decrease in testosterone level, and degeneration in testis (Boberg et al. 2011; Borch et al. 2004; Gray et al. 2000; Masutomi et al. 2003). Other studies have indicated that DINP can result in aggravated allergic dermatitis (Kang et al. 2016) and allergic asthma (Hwang et al. 2017) in mice. Studies on zebrafish demonstrated that exposure to DINP could decrease the number of fertilized eggs (Forner-Piquer et al. 2018) and lead to abnormal gonadal development and reproduction (Santangeli et al. 2017).
Diisononyl cyclohexane-1,2-dicarboxylate (DINCH)
DINCH was introduced in 2002 to replace DEHP in food contact materials. DINCH received final approval from the European Union in 2006 (Bui et al. 2016). DINCH is widely used in polyvinyl products, including children’s toys and medical devices (EFSA, 2007). The annual production of DINCH is more than 10,000 tonnes in the European market (ECHA 2021). As the use of DINCH is increasing, its level in the environment is becoming prominent (Larsson et al. 2017). In urine samples from the German Specimen Bank, cyclohexane-1,2-dicarboxylic acid monoisononyl ester (MINCH) metabolites, OH-MINCH, cx-MINCH, and oxo-MINCH were detected at concentrations of 2.09, 0.86, and 1.81 μg/L, respectively (Schutze et al. 2014). Other studies have also detected different DINCH metabolites in human urine samples (Fromme et al. 2016; Gomez Ramos et al. 2016; Kasper-Sonnenberg et al. 2019; Minguez-Alarcon et al. 2016; Schwedler et al. 2020).
Like other alternative plasticizers, the toxicity of DINCH is not properly understood. Several studies have investigated DINCH toxicity; however, the data is contradictory (Campioli et al. 2015; Campioli et al. 2019; Campioli et al. 2017; David et al. 2015; EFSA, 2007; Engel et al. 2018; Nardelli et al. 2017; Vasconcelos et al. 2019). For instance, some studies have not observed any effect on behavior, organ weight, serum chemistry (David et al. 2015), and reproduction (EFSA, 2007). However, other studies showed that DINCH and/or its metabolites caused thyroid hyperplasia and renal toxicity (EFSA, 2007), changed the expression of genes involved in lipid metabolism (Campioli et al. 2015; Campioli et al. 2019), and altered Leydig cell function, and resulted in testicular atrophy in rats (Campioli et al. 2017).
Thyroid hormone (TH) system
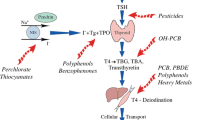
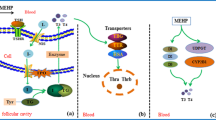
THs are produced by the thyroid gland, which sits on the neck region (Barrett et al. 2018). The name thyroid was derived from a Greek word “thyreos” for shield due to its shield-like appearance (Ellis 2011). Thyroid is the only endocrine gland that can produce and store the two THs, triiodothyronine (T3) and thyroxine (T4). T4 is the major TH secreted by the thyroid gland, whereas T3 is the main biologically active form (Dezonne et al. 2015; Izumi and Larsen 1977). From the thyroid gland, the THs are transported to different tissues. The thyroid is the main site for T4 and T3 synthesis; however, T3 synthesis also takes place in the peripheral tissues. In the target tissues, iodothyronine deiodinase (DIO) enzymes play an essential role in regulating the levels of THs. There are three types of deiodinase enzymes (DIO1, DIO2, and DIO3) that are involved in TH synthesis and regulation (Gereben et al. 2015). The deiodinases are unique enzymes as they contain selenocysteine group. DIO2 catalyzes the conversion of T4 to T3 following deiodination of the outer ring of T4 (Bianco et al. 2002; Peeters and Visser 2000). DIO1 can also generate T3 from T4 but has a lower affinity and high Km for T4 than DIO2. DIO1 and DIO3 can metabolize both T3 and T4, thereby diminishing TH signaling (Gereben et al. 2015). DIO1 and DIO3 can convert T4 to inactive T3 (rT3) by deiodination of the inner ring. DIO1 and DIO3 can further metabolize T3 (by deiodination of the inner ring) and rT3 (by deiodination of the outer ring) (Bianco et al. 2002; Peeters and Visser 2000). The tissue distribution of DIO1 and DIO3 is different, with DIO1 predominantly expressed in the liver, kidney, and thyroid, while DIO3 is largely expressed in the brain. Hence, DIO3 could be important in regulating T3 levels in the brain. On the other hand, DIO2 is expressed in the brain, esophagus, and thyroid (Peeters and Visser 2000). Environmental pollutants, including polybrominated flame retardants (PBDEs) are known to inhibit DIO2 enzymatic activity and reduce T3 synthesis in H4 glioma cells (Roberts et al. 2015). In zebrafish, a pesticide azocyclotin was shown to reduce T3 levels by altering Dio2 level (Jiao et al. 2019).
The synthesized TH exerts its action by interacting with thyroid hormone receptors (THRs; THRα and THRβ) located in the nucleus. Following activation of THRα or THRβ by TH, corepressors dissociate from THR due to conformational changes, and coactivators are recruited, and this complex drives the transcription of thyroid-regulated genes (Fig. 1) (Brent 2012). TH plays a crucial role in regulating different aspects of animal physiology, including energy homeostasis, circadian rhythm, cellular growth, and development (Baksi and Pradhan 2021; Brent 2012; Ikegami et al. 2019; Mullur et al. 2014). TH also influences brain development, as it is involved in neurogenesis, neuronal migration, neuronal and glial differentiation, myelination, and synaptogenesis (Lima et al. 1998; Manzano et al. 2007; Martinez-Galan et al. 1997; Schoonover et al. 2004). Alteration in TH, either low (hypothyroidism) or high (hyperthyroidism), can cause problems related to bone (osteoporosis), brain (cognition, visual attention, visual processing, motor skills, language, and memory skills), fatigue, and temperature intolerance (Williams 2008). Neuro-psychiatric disorders such as schizophrenia, bipolar disorder, anxiety, and depression are also associated with abnormal TH levels (Nandi-Munshi and Taplin 2015; Noda 2018).
Thyroid Hormone Signaling and Regulation. Thyroid hormones (THs), triiodothyronine (T3) and thyroxine (T4,) enter the target cell through transporters present on the cell membrane. Once inside the cell, T4 gets converted to T3, primarily by type II iodothyronine deiodinase (DIO2) enzyme and to a low extent by type I iodothyronine deiodinase (DIO1). T4 and T3 are converted to inactive metabolites by DIO1 and type III iodothyronine deiodinase (DIO3). TH can bind to thyroid hormone receptors (THRs) in the nucleus and carry out gene expression. The THR makes a complex with retinoid X receptor (RXR) and binds to the thyroid response element (TRE) on the upstream region of the gene transcription start site. In a resting or uninduced state, the receptor complex binds to corepressor, which blocks the gene transcription. The binding of TH to THR leads to a conformational change of THR, which releases corepressor, and coactivators bind to start the transcription of TH response genes. The figure was generated using Biorender software
Thyroid-related problems, including brain functions, are on the rise, and this could be attributed to the increasing level of environmental pollutants (Mughal et al. 2018). However, there is limited information about the effects of plasticizers on thyroid disruption and the molecular mechanisms of toxicity. Filling this knowledge gap will help understand the impacts of pollutants on physiological functions and screen new emerging chemicals for their disrupting properties. The health effects associated with phthalates and/or their metabolites on model organisms are summarized in Table 1.
The impacts of plasticizers on thyroid system
Impacts on aquatic animals
Environmental pollutants, including plasticizers, can end up in the water bodies, and aquatic animals could be at greater risk. Hence, it is important to understand the toxicity of pollutants in different aquatic model systems. Many studies highlighting plasticizer’s toxicity on the TH system have been published. Exposure to the DEHP metabolite, MEHP (200 µg/L), reduced T4 level in zebrafish larvae, while the level of T3 was increased. Genes involved in hypothalamus-pituitary-thyroid (HPT) axis, nis, pared box 8 (pax8), NK2 homeobox 1 (nkx2.1), thyroglobulin (tg), ttr, dio1, dio2, and UDP glucuronosyltransferase 1 family polypeptide a3 isoform 1 (ugt1ab) were affected at doses of 8–200 µg/L MEHP (Zhai et al. 2014). In another study using a similar experimental setup, Jia et al. (2016) showed that exposure of zebrafish larvae to 400 µg/L of DEHP can increase T4 and T3 levels (Jia et al. 2016). In the same study, DEHP altered the expression of several thyroid-related genes such as thyroid-stimulating hormone beta (tshβ), corticotropin-releasing hormone (crh), nkx2.1, tg, and dio2 in a dose-dependent manner (Jia et al. 2016). MEHP is known to be more toxic than DEHP (Pollack et al. 1985); hence, the higher toxicity of MEHP on the TH system is not surprising.
In an in vitro and in vivo study, Sugiyama et al. (2005) analyzed the effects of phthalates on the thyroid system. Using the luciferase assay, it was found that dicyclohexyl phthalate (DCHP), benzyl butyl phthalate (BBP), and DBP can inhibit T3 activity with IC50 of 11 ± 3 µM, 40 ± 6 µM and 39 ± 1 µM, respectively. In in vivo experiment, Xenopus laevis tadpoles immersed in buffer with 2-nM T3 alone or in combination with phthalates showed that co-treatment with BBP can significantly downregulate T3 dependent induction of THR beta (THRβ) gene (Sugiyama et al. 2005). In a similar study, African clawed frog tadpoles exposed to 2, 10, or 15 mg/L DBP or mono butyl phthalate (MBP) for 21 days showed that the two phthalates at all the concentrations can alter the expression of several thyroid-related genes, including THRβ, retinoid X receptor gamma (RXRγ), TSHα, and TSHβ. An in vitro analysis further showed that DBP and MBP could induce the interaction between silencing mediator for retinoid and thyroid hormone receptors (SMRT) and THR in a dose-dependent manner. The authors highlighted that DBP and MBP have a potential to disrupt thyroid activity (Shen et al. 2011).
Impacts on rodents
Several studies have been conducted in rodent models to analyze the effects of plasticizers on the TH system. DEHP exposure on rats (oral gavage, 250, 500, and 750 mg/kg/day) resulted in alteration of genes involved in TH signaling (Liu et al. 2015). TSH receptor (TSHr) expression was significantly downregulated in response to 500 and 750 mg/kg/day DEHP, while thyrotropin-releasing hormone receptor (TRHr) was significantly upregulated in a dose-dependent manner (Liu et al. 2015). Histological analysis indicated that DEHP could alter thyroid follicular cell hypertrophy and hyperplasia in 500 and 750 mg/kg/day doses. Total T4 (TT4) and free T4 (FT4) were reduced by 500 and 750 mg/kg/day doses; however, no effect was observed on TSH level. Total T3 (TT3) and free T3 (FT3) were also reduced by 750 mg/kg/day dose, while only TT3 was reduced by 500 mg/kg/day dose. Serum level of thyroid peroxidase (TPO) was reduced by both 500 and 750 mg/kg/day doses, while NIS was reduced by only 750 mg/kg/day dose (Liu et al. 2015). mRNA and protein levels of TTR were downregulated by all the doses of DEHP. Based on the findings, the authors argued that the DEHP-mediated alteration of TH could be due to changes in biosynthesis, transformation, transport, and metabolism of TH (Liu et al. 2015).
Other studies have also investigated whether there was an association between phthalates and thyroid profiles. Baralic et al. (2020) showed that rats orally dosed for 27 days with DEHP (50 mg/kg/day), DBP (50 mg/kg), BPA (25 mg/kg), and a mixture of these three chemicals can alter thyroid profile. Serum content of T3 and T4 showed a negative association with DBP and BPA (Baralic et al. 2020). Supporting this, Wu et al. (2017a, b) found that oral exposure to DBP (50 mg/kg/day) for 5 weeks can decrease T3 levels in female Wistar rats (Wu et al. 2017b). In a recent study, Wu et al. (2021) analyzed the effects of DEHP (150, 300, and 600 mg/kg) on the HPT axis using a total of 40 Wistar rats (2 weeks old) upon intragastric administration for 90 days. Although the levels of THs, FT3, and FT4 did not change in 150 mg/kg/day exposure group, the levels of T4 and FT3 were significantly decreased in response to 300 mg/kg/day exposure group. The levels of T3 and TSH in the 600 mg/kg/day exposure group were also significantly altered. DEHP exposure also led to histological changes in the thyroid gland. Besides, DEHP resulted in altered expression of genes and proteins involved in TSH/TSHr signaling pathways. Altogether, the authors concluded that DEHP could disrupt TH homeostasis through different mechanisms (Wu et al. 2021). In a study, chronic exposure to BPA (200 mg/kg bw/day for 35 days) resulted in significantly decreased serum levels of T3 and T4 accompanied by an increased serum TSH level in rats. The authors supported their findings through histological analysis indicating severe pathological changes in the thyroid tissue of BPA-treated rats (Mohammed et al. 2020).
Analysis of six plasticizers using rat thyroid follicular cells, Wenzel et al. (2005) showed that DEHP, di-isodecyl phthalate (DIDP), di-octyl phthalate (DOP), and DINP can enhance iodide uptake by regulating NIS. Other plasticizers, including BBP and DBP, showed no effect on iodide uptake (Wenzel et al. 2005). Analysis of DEHP and di-n-hexyl phthalate (DnHP) on male rats showed alteration in thyroid tissue. Exposure to phthalates, DEHP, and DnHP alone or in combination (10 000 ppm concentration for both) resulted in reduction of follicular cells and increase in the number of follicular cells with a columnar appearance, a sign of hyperactivity (Howarth et al. 2001). Interestingly, the effect of DnHP was stronger in thyroid tissue but did not alter other parameters, such as liver weight, induction of fatty acid oxidation, and CYP4A1 like DEHP (Howarth et al. 2001).
The physiology in organisms, including the thyroid system and TH levels, differ in females and males. This could result in sex-specific effects of environmental pollutants, and revealing this may help better understand the toxicity of pollutants. Several studies have demonstrated that phthalates have sex-specific effects on TH activities. Tassinari et al. (2021) orally exposed juvenile rats to various concentrations of DEHP (0, 9, 21, and 48 mg/kg bw/day) for 28 days and analyzed different parameters. Histomorphometric analysis indicated that DEHP demonstrated no significant change in male rats, while thyroid follicle areas, colloid areas, and number of cells in follicular epithelium significantly increased in female rats in response to 9 and 48 mg/kg bw/day groups. Besides, epithelium areas significantly increased in female rats in 9 mg/kg bw/day group. TSH gene expression was significantly downregulated in females in 48 mg/kg bw/day group. Altogether, it was suggested that DEHP could alter thyroid homeostasis in immature rats (Tassinari et al. 2021). TH signaling shows sex-specific differences (Baksi and Pradhan 2021), and plasticizer-mediated sex-specific toxicity (Tassinari et al. 2021) could be an important indication that both males and females are needed to assess the toxicity of plasticizers on the TH system.
Hypothalamus and pituitary are important for regulating thyroid function, and the released TH from the thyroid can have negative feedback on them (Fig. 2). This loop, the hypothalamic-pituitary-thyroid (HPT) axis, is critical for maintaining different physiological functions. Plasticizers are suggested to disrupt the HPT axis, and few studies have highlighted this. In a study, juvenile Wistar rats were exposed to 0, 5, 50, and 500 mg/kg/day DEHP for 28 days by oral gavage to analyze the effect of DEHP on HPT axis. It was demonstrated that higher concentrations of DEHP can decrease protein levels of TRH in the hypothalamus, increase protein and mRNA levels of TRHr in the pituitary, and decrease mRNA level of TSHr in the thyroid. The authors concluded that DEHP could interfere with the thyroid homeostasis in juvenile rats (Sun et al. 2018). Dong et al. (2017) investigated the effect of various concentrations of DEHP (oral gavage of 150, 300, and 600 mg/kg/day for 3 and 6 months) on THs in Wistar rats (128 rats) at the transcript and protein levels. The authors observed that DEHP can alter mRNA and protein levels of TRHr, TSHβ, TSHr, TPO, thyroid transcription factor 1 (TTF-1), and TG. The authors suggested that exposure to DEHP could affect TH levels by altering HPT axis of the body through TSH/TSHr signaling (Dong et al. 2017).
Schematic representation of thyroid signaling and potential mechanisms of toxicity of different plasticizers. The hypothalamus and pituitary regulate the thyroid gland. Hypothalamus secretes thyrotropin releasing hormone (TRH) that acts on pituitary, resulting in the release of thyroid stimulating hormone (TSH). TSH acts on the thyroid to induce the production of THs (thyroxine: T4 and triiodothyronine: T3), which in turn act on the hypothalamus and pituitary for negative feedback regulation. T4 and T3 produced in the thyroid gland reach other tissues through the circulatory system. TH can bind to thyroid hormone receptor (THRs) in the nucleus and regulate gene expression. Plasticizers are known to impact different steps of TH signaling. For instance, they can bind to THRs, retinoid X receptor (RXR), thyroid stimulating hormone receptor (TSHr), thyroglobulin (TG). Plasticizers can also influence TH signaling by regulating enzymes, including DIO1, DIO2 and DIO3 involved in TH synthesis and metabolism. Although plasticizers have been shown to impact transcript levels of deiodinase enzymes, it is not known if they can alter TH synthesis by directly affecting deiodinase enzyme activity at the protein level
Impacts on humans
Humans can be exposed to plasticizers through multiple routes, including oral, inhalation, and dermal. It is likely that humans are getting exposed to plasticizers from the day-to-day items surrounding them. In 2011, there was a food scandal in Taiwan where the two plasticizers DEHP and DINP were purposely added by the companies in their items. Of the 965 contaminated products, 206 were exported to 22 countries (Yang et al. 2013). This indicates that humans may be exposed to phthalates continuously through various routes and sources. Phthalates are rapidly degraded into their metabolites and excreted in urine and serum upon exposure (Hauser and Calafat 2005). Phthalates may change physiological conditions in humans directly or through their metabolites. Several epidemiological studies have analyzed the association of phthalate exposure with the human thyroid system and hormone levels (Table 2). Wu et al. (2013) analyzed 60 children of 4–6 years old upon exposure to DEHP (29 high dose, 23 low dose, and 8 non-exposed) and found that the serum TSH levels were significantly lower in the high- and low-dose groups. However, T3, T4, and FT4 levels were not different from the non-exposed group. Interestingly, 6-month follow-up study showed no change in TSH, but T3 level was significantly altered in the high dose group (n = 13). In this study, it was indicated that FT3 is more relevant for biological action than T3 (Wu et al. 2013). Later, the same group performed a study with 250 participants that were exposed to plasticizers. However, no significant association was found with the serum TSH, T4, T3, and FT4 levels (Tsai et al. 2016). Similar results were obtained from another study that was performed on 329 teenagers (12–19 years) and 1346 adults (above 19 years) (Meeker and Ferguson, 2011). The authors found that the urinary DEHP metabolites levels in adults were inversely associated with T3, T4, FT4, and TG, whereas a positive association was observed with TSH. Interestingly, DEHP metabolite showed a positive association with T3 and TSH in teenagers (Meeker and Ferguson, 2011). Since thyroid-related problems are known to show sex-specific and age-dependent prevalence (Cho et al. 2018; Gietka-Czernel 2017), this study further encourages the analysis of pollutant’s impacts on different age groups and sex. Meeker et al. (2007) analyzed urine samples from 408 men (18–55 years old) and measured phthalate metabolites, T4, T3, and TSH levels. MEP was detected in all the 408 men, and it showed negative and positive association with TT3 and TSH, respectively. The authors noted a weak inverse association between MEHP and FT3 (Meeker et al. 2007). As the authors also pointed out, the drawback of this study was that TT4 and FT3 levels were not measured.
Huang et al. (2017b) performed a study with 279 Taiwanese adults (≥ 18 years old) and 79 minors (< 18 years old) and investigated the association between phthalate exposure and thyroid function in 2013. The authors showed that the levels of urinary mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP) and the total of urinary DEHP metabolites were inversely correlated with T4 levels in adults, while FT4 levels were inversely correlated with urinary MEHP and mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP) levels, and positively correlated with urinary MEP. Meanwhile, FT4 was positively correlated with urinary mono benzyl phthalate (MBzP) levels in minors (Huang et al. 2017b). This shows that phthalate exposure could affect thyroid function differently in adult and young populations. This differential regulation could be due to differences in metabolic activity.
Boas et al. (2010) analyzed the concentration of phthalate metabolites in urine samples of 845 Danish children aged 4–9 years to determine whether these metabolites are associated with thyroid functions. The authors detected all the 12 phthalate metabolites in all urine samples with the highest concentration of MBP. They also determined an inverse correlation between metabolites and serum levels of FT3 and TT3 in girls (Boas et al. 2010). This further suggests that there could be gender-specific effects of phthalate metabolites on TH system. It has been indicated that women show 5–20 times higher susceptibility to thyroid-related problems (Gietka-Czernel 2017). The underlying mechanisms are not clear and the differential action of pollutants could also be a contributing factor. Wu et al. (2017a) investigated the association between urinary concentrations of eight mono-phthalate metabolites (mPAEs) and thyroid function in China with 216 children aged 5–7 years. The authors found that mPAEs concentrations in children from urban areas were higher than those from rural areas. Most of the mPAEs positively correlated with FT3 and FT4 suggesting that phthalate exposure in children could affect TH (Wu et al. 2017a). In another study with 317 participants, including 165 workers engaged in waste plastic recycling and 152 farmers, it was shown that urinary levels of phthalates, particularly MBzP, MEHHP, and monooctyl phthalate (MOP) were significantly higher in workers compared to the controls (Wang et al. 2018). In contrast to a previous study (Boas et al. 2010), the phthalate metabolites were positively associated with T3 or T3/T4 ratio in all the participants. A non-monotonic dose–response relation between urinary phthalate metabolites and serum levels of TH, including mono-methyl phthalate (MMP) and T3 or T3/T4 ratio, and MEP and T3/T4 ratio was also demonstrated (Wang et al. 2018).
To show the association between phthalates and thyroid function, Park et al. (2017) measured the levels of several phthalate (DEHP, BBP, and DBP) metabolites in urine samples, and T3, T4, and TSH in serum samples. In this study, a large sample size of the Korean adult population involving 6003 participants during 2012–2014 was enrolled. The level of phthalate metabolites was associated with decreased T3 and T4 in urine samples, and increased TSH levels in serum samples. The authors showed that the level of MEHHP metabolite in urine inversely correlated with T4 in males, while MBzP and mono-n-butyl phthalate (MnBP) metabolites inversely correlated with T3 and TSH in females. Altogether, the authors suggested that exposure to phthalates have a potential to alter TH balance in adults (Park et al. 2017).
Exposure to phthalates during pregnancy may alter TH system, resulting in adverse effects on pregnancy, birth outcomes, and development of children (Villanger et al. 2020). The maternal urinary phthalate metabolites and TH levels have also been analyzed during pregnancy in different studies. Romano et al. (2018) measured the concentration of nine phthalate metabolites in urine samples from women at around 16–26 weeks of gestation, while they measured TSH, FT4, and TT4 and T3 in 202 maternal serums at 16 weeks of gestation and in 276 cord serums at delivery. A tenfold increase in maternal urinary MEP metabolite at 16 weeks of gestation was associated with decreased in maternal TT4. On the other hand, a tenfold increase in maternal urinary MBzP metabolite at both 16 and 26 weeks of gestation correlated with decreased cord serum TSH. This indicated that phthalate indexes in mothers and newborns negatively correlate with maternal serum TT4 and cord serum TSH, respectively (Romano et al. 2018). In a similar study, urine and serum samples were collected at 16 and 26 weeks of gestation in Northern Puerto Rico. Significant negative correlations were observed between mono-3-carboxypropyl phthalate (MCPP) and FT3 at 16 and 26 weeks of gestation, as well as DEHP and FT4 at 26 weeks of gestation. It was suggested that phthalate urinary metabolites could change maternal serum thyroid levels in a time-dependent exposure during gestation (Johns et al. 2015). Johns et al. (2016) further performed repeated measures of urinary phthalate metabolites and plasma TH levels in 439 pregnant women (116 cases and 323 controls) and demonstrated that phthalate metabolites negatively correlated with TSH and positively correlated with free and total TH. It was indicated that the time of exposure during gestation could affect the level and direction of these correlations (Johns et al. 2016). Derakhshan et al. (2021) analyzed the association of thyroid function with phthalate exposure in 1996 pregnant women. The authors found that higher DEHP and DINP metabolites were correlated with a lower FT4 and TT4, respectively. They also showed that higher concentrations of DBP and BBP metabolites were associated with lower T4/T3, higher FT4/TT4, and higher FT3/TT3. Higher DINCH metabolites were also found to correlate with higher TT3 (Derakhshan et al. 2021).
Yao et al. (2016) showed a link between phthalate exposure during the first trimester and TH in pregnant women and their newborns. In this study, the level of TH was measured in maternal and cord sera, while the level of seven phthalates was determined in urea. It was demonstrated that increase in MEHP and MEHHP associated with a decrease in maternal TT4, while the same metabolites were negatively and positively associated with maternal FT4 and maternal TSH, respectively. The authors did not observe any association between phthalate metabolites and TH in cord serum. Taken together, the authors suggested that exposure to phthalates during the first trimester could affect maternal TH levels (Yao et al. 2016). Huang et al. (2007) investigated 76 urine and serum samples from Taiwanese pregnant women in the second trimester to analyze whether there is an association between phthalate exposure and TH. The authors found that MBP, MEP, and MEHP were the predominant metabolites with median levels of 81.8, 27.7, and 20.6 ng/ml, respectively. They also determined a significant negative association between T4, FT4, and urinary MBP. Although DBP altered the thyroid function, the mechanism of action remains unclear (Huang et al. 2007).
The association between phthalates and mother–child pairs and children has also been investigated. Huang et al. (2017a) performed a meta-analysis with 181 mother–child pairs in central Taiwan and measured T3, T4, FT4, and TSH in children, as well as phthalate metabolites in urine samples. The authors showed that maternal DEHP metabolites, MEHHP and MEOHP, were negatively associated with T3 and T4 levels in boys. On the other hand, in girls, FT4 levels were negatively correlated with maternal urinary MEP, maternal urinary MBzP, and children’s urinary MEHP. The authors suggested that exposure to phthalates in early life could suppress TH levels in young children (Huang et al. 2017a). Kuo et al. (2015) performed a study on 148 Taiwanese maternal and infant pairs by collecting urine and blood samples in the third trimester of pregnant women and their cord blood samples at delivery. Among the detected nine phthalate metabolites, the urinary MBzP was found to be inversely associated with serum TSH in cord blood, suggesting that the parental compound of MBzP, BBP, could alter TSH function in newborns (Kuo et al. 2015). Weng et al. (2017) analyzed the effects of phthalates on thyroid function in 189 Taiwanese children based on gender and found a positive association between T4 and DEHP metabolites, MEHP, MEOHP, and MEHHP in girls, while in boys, there was a positive correlation between FT3 and DBP metabolites, mono-i-butyl phthalate (MiBP), and MnBP. It was also demonstrated that higher concentrations of DEHP have higher impacts on FT3 in boys. The authors suggested that phthalate metabolites could cause gender-specific effects on thyroid function (Weng et al. 2017).
Thyroid cancer is the most diagnosed cancer type (Miao et al. 2020) and several studies have suggested that the endocrine-disrupting chemicals may imbalance the TH system, leading to adverse outcomes, including cancer (Alsen et al. 2021; Marotta et al. 2020; Miao et al. 2020). Since several phthalates have been shown to have endocrine disruptive effects (Marotta et al. 2020; Villanger et al. 2020), it is critical to reveal their potential contributions to the etiology of thyroid disorders and cancers. Many studies have investigated the association between phthalates and thyroid cancer. Marotta et al. (2019) analyzed the association between DEHP and thyroid cancer development on 55 patients, 27 with a diagnosis of benign thyroid nodules, and 28 suffering from differentiated thyroid cancer. The authors observed a correlation between serum levels of DEHP and malignancy in dose-independent manner. However, this correlation was not mediated by higher TSH levels (Marotta et al. 2019). The authors indicated that the existence of DEHP in serum could result in a more than 14 times higher risk of developing differentiated thyroid cancer (Marotta et al. 2019). In another study, the role of urinary phthalate metabolites on the risk of thyroid cancer was investigated using sex-matched 44 thyroid cancer, 138 benign nodule patients, and 144 healthy adults from Wuhan, China (Liu et al. 2020). The authors demonstrated a positive correlation between three phthalate metabolites, MMP, MEHHP, and MEHP, and thyroid cancer. Meanwhile, urinary MBP and MBzP were found to be negatively correlated with thyroid cancer. The authors also indicated a gender-specific relation between phthalate metabolites and thyroid cancer, as MEHP significantly correlated in females but not in males (Liu et al. 2020). Miao et al. (2020) analyzed the link between urinary phthalate metabolites and papillary thyroid cancer and a positive correlation between DEHP metabolites and papillary thyroid cancer was observed. Although the results require confirmation, the authors indicated that phthalate exposure affects papillary thyroid cancer development (Miao et al. 2020). Taken together, although the summarized studies indicate a positive correlation between phthalates and thyroid cancer, the available data cannot reveal a definitive association between chemical exposure and disease development due to a lack of evidence (Marotta et al. 2020).
In vitro and in silico studies
Other studies have investigated phthalate exposure and thyroid functions using meta-analysis and in vitro and in silico methods. In a meta-analysis study with 13 articles, Kim et al. (2019) demonstrated an inverse association between urinary MEHP and MEHHP concentrations and TT4, while urinary 5-oxo-MEHP metabolite concentration was positively associated with thyrotropin. It was concluded that exposure to DEHP metabolites could be significantly correlated with the function of the HPT axis (Kim et al. 2019).
In vitro analysis using monkey fibroblast–derived CV-1 cells, Shen et al. (2009) showed that DEHP, DBP, and MBP can bind to TH receptor as antagonists with IC50 of 13 µM, 2.7 µM, and greater than 100 µM, respectively (Shen et al. 2009). Using the same cell line (CV-1), Ibhazehiebo and Koibuchi (2011) analyzed the impacts of DEHP exposure on THR-mediated gene expression and found that the low dose of DEHP (10–7 M) can suppress 30% of THR-mediated transcription. It was suggested that DEHP is a non-competitive inhibitor of THR as no dose-dependent activity was observed (Ibhazehiebo and Koibuchi 2011). Using in vitro and in silico approaches, Kambia et al. (2021) compared the effects of DEHP and DEHT metabolites on THRs. The in vitro assay (rat pituitary tumor cell line GH3) showed that DEHT metabolite MEHT has an antagonistic effect at below cytotoxic doses (2–5 µg/mL), while MEHP was found to be partial agonist between 10 and 20 µg/mL. It was also demonstrated that 5-OH-MEHP primarily acts as an agonist at a concentration of 0.2 µg/mL, and at higher doses, it shows synergetic effect with T3. These in vitro data were also confirmed by in silico docking results (Kambia et al. 2021).
Conclusion
TH regulates almost every part of the body, and it is implicated in a wide range of physiological functions. From the available data, it is evident that plasticizers negatively regulate the TH system. This is a matter of concern as the production of plasticizers is expected to increase and new alternatives are also introduced whose toxicity is not fully elucidated. Plasticizers could have a long-lasting impact on organisms by affecting their TH homeostasis. TH action can be regulated at different levels (Fig. 2); hence, plasticizers’ mechanisms of action to impact the TH system could be diverse. Plasticizers could demonstrate positive and/or negative association with serum levels of T3 and T4 and alter hormone levels, including TSH and TRH in a sex-specific manner. Plasticizers could also affect the genes and proteins involved in the TH system and cause thyroid follicular cell hypertrophy and hyperplasia. The available data also indicate age-dependent impacts of plasticizers on the TH system as several plasticizers were detected in urine and serum of children and adults at different levels. Since plasticizers affect the TH system, they can be classed under endocrine disruptors. Hence, a thorough analysis of old and new emerging plasticizers in the regulation of the thyroid system is required.
Data availability
Not applicable.
Code availability
Not applicable.
References
Agency (2010) EC. Evaluation of new scientific evidence concerning the restrictions contained in Annex XVII to Regulation (EC) No. 1907/2006 (REACH): review of new available information for di-’isononyl’Phthalate (DINP)
Ait Bamai Y, Araki A, Kawai T, Tsuboi T, Saito I, Yoshioka E et al (2014) Associations of phthalate concentrations in floor dust and multi-surface dust with the interior materials in Japanese dwellings. Sci Total Environ 468–469:147–157
Alsen M Sinclair C Cooke P Ziadkhanpour K Genden E van Gerwen M. Endocrine Disrupting chemicals and thyroid cancer: an overview. Toxics 2021; 9
Andrade AJ, Grande SW, Talsness CE, Gericke C, Grote K, Golombiewski A et al (2006) A dose response study following in utero and lactational exposure to di-(2-ethylhexyl) phthalate (DEHP): reproductive effects on adult male offspring rats. Toxicology 228:85–97
Baksi S, Pradhan A (2021) Thyroid hormone: sex-dependent role in nervous system regulation and disease. Biol Sex Differ 12:25
Barakat R, Lin PP, Rattan S, Brehm E, Canisso IF, Abosalum ME et al (2017) Prenatal Exposure to DEHP Induces Premature Reproductive Senescence in Male Mice. Toxicol Sci 156:96–108
Baralic K, Buha Djordjevic A, Zivancevic K, Antonijevic E, Andelkovic M, Javorac D, et al. Toxic effects of the mixture of phthalates and bisphenol a-subacute oral toxicity study in Wistar rats. Int J Environ Res Public Health 2020; 17.
Barnabe S, Beauchesne I, Cooper DG, Nicell JA (2008) Plasticizers and their degradation products in the process streams of a large urban physicochemical sewage treatment plant. Water Res 42:153–162
Barrett KE Barman SM Boitano S Brooks HL. The thyroid gland. Ganong’s Review of Medical Physiology, 25e. McGraw-Hill Education, New York, NY, 2018.
Bauer MJ, Herrmann R (1997) Estimation of the environmental contamination by phthalic acid esters leaching from household wastes. Sci Total Environ 208:49–57
Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR (2002) Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev 23:38–89
Bilal M, Iqbal HMN, Barcelo D (2019) Mitigation of bisphenol A using an array of laccase-based robust bio-catalytic cues - A review. Sci Total Environ 689:160–177
Blair JD, Ikonomou MG, Kelly BC, Surridge B, Gobas FA (2009) Ultra-trace determination of phthalate ester metabolites in seawater, sediments, and biota from an urbanized marine inlet by LC/ESI-MS/MS. Environ Sci Technol 43:6262–6268
Boas M, Frederiksen H, Feldt-Rasmussen U, Skakkebaek NE, Hegedus L, Hilsted L et al (2010) Childhood exposure to phthalates: associations with thyroid function, insulin-like growth factor I, and growth. Environ Health Perspect 118:1458–1464
Boas M, Feldt-Rasmussen U, Main KM (2012) Thyroid effects of endocrine disrupting chemicals. Mol Cell Endocrinol 355:240–248
Boberg J, Christiansen S, Axelstad M, Kledal TS, Vinggaard AM, Dalgaard M et al (2011) Reproductive and behavioral effects of diisononyl phthalate (DINP) in perinatally exposed rats. Reprod Toxicol 31:200–209
Borch J, Ladefoged O, Hass U, Vinggaard AM (2004) Steroidogenesis in fetal male rats is reduced by DEHP and DINP, but endocrine effects of DEHP are not modulated by DEHA in fetal, prepubertal and adult male rats. Reprod Toxicol 18:53–61
Brent GA (2012) Mechanisms of thyroid hormone action. J Clin Invest 122:3035–3043
Bui TT, Giovanoulis G, Cousins AP, Magner J, Cousins IT, de Wit CA (2016) Human exposure, hazard and risk of alternative plasticizers to phthalate esters. Sci Total Environ 541:451–467
Campioli E, Duong TB, Deschamps F, Papadopoulos V (2015) Cyclohexane-1,2-dicarboxylic acid diisononyl ester and metabolite effects on rat epididymal stromal vascular fraction differentiation of adipose tissue. Environ Res 140:145–156
Campioli E, Lee S, Lau M, Marques L, Papadopoulos V (2017) Effect of prenatal DINCH plasticizer exposure on rat offspring testicular function and metabolism. Sci Rep 7:11072
Campioli E, Lau M, Papadopoulos V (2019) Effect of subacute and prenatal DINCH plasticizer exposure on rat dams and male offspring hepatic function: The role of PPAR-alpha. Environ Res 179:108773
Caresana. Plasticizers Market Report https://www.ceresana.com/en/market-studies/chemicals/plasticizers/ceresana-market-study-plasticizers.html 2019.
Chen H, Zhang W, Rui BB, Yang SM, Xu WP, Wei W (2016) Di(2-ethylhexyl) phthalate exacerbates non-alcoholic fatty liver in rats and its potential mechanisms. Environ Toxicol Pharmacol 42:38–44
Chen H, Feng W, Chen K, Qiu X, Xu H, Mao G et al (2019) Transcriptomic analysis reveals potential mechanisms of toxicity in a combined exposure to dibutyl phthalate and diisobutyl phthalate in zebrafish (Danio rerio) ovary. Aquat Toxicol 216:105290
Cho BA, Yoo SK, Song YS, Kim SJ, Lee KE, Shong M et al (2018) Transcriptome network analysis reveals aging-related mitochondrial and proteasomal dysfunction and immune activation in human thyroid. Thyroid 28:656–666
David RM, White RD, Larson MJ, Herman JK, Otter R (2015) Toxicity of Hexamoll((R)) DINCH((R)) following intravenous administration. Toxicol Lett 238:100–109
Derakhshan A, Shu H, Broeren MAC, Lindh CH, Peeters RP, Kortenkamp A et al (2021) Association of phthalate exposure with thyroid function during pregnancy. Environ Int 157:106795
Dezonne RS, Lima FR, Trentin AG, Gomes FC (2015) Thyroid hormone and astroglia: endocrine control of the neural environment. J Neuroendocrinol 27:435–445
Dobrzynska MM (2016) Phthalates widespread occurrence and the effect on male gametes Part 2 The effects of phthalates on male gametes and on the offspring. Rocz Panstw Zakl Hig 67:209–21
Dong X Dong J Zhao Y Guo J Wang Z Liu M et al. 2017 Effects of long-term in vivo exposure to di-2-ethylhexylphthalate on thyroid hormones and the TSH/TSHR signaling pathways in Wistar rats. Int J Environ Res Public Health; 14
Duty SM, Ackerman RM, Calafat AM, Hauser R (2005) Personal care product use predicts urinary concentrations of some phthalate monoesters. Environ Health Perspect 113:1530–1535
EFSA. 2007 Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to a 16th list of substances for food contact materials.; 5: 515
Ellis H (2011) The early days of thyroidectomy. J Perioper Pract 21:215–216
Ema M, Amano H, Itami T, Kawasaki H (1993) Teratogenic evaluation of di-n-butyl phthalate in rats. Toxicol Lett 69:197–203
Engel A, Buhrke T, Kasper S, Behr AC, Braeuning A, Jessel S et al (2018) The urinary metabolites of DINCH((R)) have an impact on the activities of the human nuclear receptors ERalpha, ERbeta, AR. Pparalpha and PPARgamma Toxicol Lett 287:83–91
European Chemicals Agency – Information on Chemicals (2021) Available: https://echa.europa.eu/substanceinformation/-/substanceinfo/100.103.017
Fatoki O, Noma A (2002) Solid phase extraction method for selective determination of phthalate esters in the aquatic environment. Water Air Soil Pollut 140:85–98
Fatoki O, Ogunfowokan A (1993) Determination of phthalate ester plasticizers in the aquatic environment of southwestern Nigeria. Environ Int 19:619–623
Fatoki OS, Vernon F (1990) Phthalate esters in rivers of the Greater Manchester area. UK Sci Total Environ 95:227–232
Forner-Piquer I, Santangeli S, Maradonna F, Rabbito A, Piscitelli F, Habibi HR et al (2018) Disruption of the gonadal endocannabinoid system in zebrafish exposed to diisononyl phthalate. Environ Pollut 241:1–8
Foster PM, Thomas LV, Cook MW, Gangolli SD (1980) Study of the testicular effects and changes in zinc excretion produced by some n-alkyl phthalates in the rat. Toxicol Appl Pharmacol 54:392–398
Fromme H, Kuchler T, Otto T, Pilz K, Muller J, Wenzel A (2002) Occurrence of phthalates and bisphenol A and F in the environment. Water Res 36:1429–1438
Fromme H, Lahrz T, Piloty M, Gebhart H, Oddoy A, Rüden H (2004) Occurrence of phthalates and musk fragrances in indoor air and dust from apartments and kindergartens in Berlin (Germany). Indoor Air 14:188–195
Fromme H, Schutze A, Lahrz T, Kraft M, Fembacher L, Siewering S et al (2016) Non-phthalate plasticizers in German daycare centers and human biomonitoring of DINCH metabolites in children attending the centers (LUPE 3). Int J Hyg Environ Health 219:33–39
Gereben B, McAninch EA, Ribeiro MO, Bianco AC (2015) Scope and limitations of iodothyronine deiodinases in hypothyroidism. Nat Rev Endocrinol. 11:642–52
Gietka-Czernel M (2017) The thyroid gland in postmenopausal women: physiology and diseases. Prz Menopauzalny 16:33–37
Gimeno P, Thomas S, Bousquet C, Maggio AF, Civade C, Brenier C et al (2014) Identification and quantification of 14 phthalates and 5 non-phthalate plasticizers in PVC medical devices by GC-MS. J Chromatogr B Analyt Technol Biomed Life Sci 949–950:99–108
Godwin AD. 2017 Plasticizers. Applied plastics engineering handbook. Elsevier, , pp. 533–553
Gomez Ramos MJ, Heffernan AL, Toms LML, Calafat AM, Ye X, Hobson P et al (2016) Concentrations of phthalates and DINCH metabolites in pooled urine from Queensland. Australia Environ Int 88:179–186
Graham PR (1973) Phthalate ester plasticizers–why and how they are used. Environ Health Perspect 3:3–12
Grande SW, Andrade AJ, Talsness CE, Grote K, Chahoud I (2006) A dose-response study following in utero and lactational exposure to di(2-ethylhexyl)phthalate: effects on female rat reproductive development. Toxicol Sci 91:247–254
Gray LE Jr, Ostby J, Furr J, Price M, Veeramachaneni DN, Parks L (2000) Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol Sci 58:350–365
Guo Y, Wu Q, Kannan K (2011) Phthalate metabolites in urine from China, and implications for human exposures. Environ Int 37:893–898
Hauser R, Calafat AM (2005) Phthalates and human health. Occup Environ Med 62:806–818
He Y, Wang Q, He W, Xu F (2019) The occurrence, composition and partitioning of phthalate esters (PAEs) in the water-suspended particulate matter (SPM) system of Lake Chaohu. China Sci Total Environ 661:285–293
Heudorf U, Mersch-Sundermann V, Angerer J (2007) Phthalates: toxicology and exposure. Int J Hyg Environ Health 210:623–634
Horn O, Nalli S, Cooper D, Nicell J (2004) Plasticizer metabolites in the environment. Water Res 38:3693–3698
Howarth JA, Price SC, Dobrota M, Kentish PA, Hinton RH (2001) Effects on male rats of di-(2-ethylhexyl) phthalate and di-n-hexylphthalate administered alone or in combination. Toxicol Lett 121:35–43
Huang PC, Kuo PL, Guo YL, Liao PC, Lee CC (2007) Associations between urinary phthalate monoesters and thyroid hormones in pregnant women. Hum Reprod 22:2715–2722
Huang HB, Chuang CJ, Su PH, Sun CW, Wang CJ, Wu MT et al (2017) Prenatal and childhood exposure to phthalate diesters and thyroid function in a 9-year follow-up birth cohort study: Taiwan Maternal and Infant Cohort Study. Epidemiology 28(Suppl 1):S10–S18
Huang HB, Pan WH, Chang JW, Chiang HC, Guo YL, Jaakkola JJ et al (2017) Does exposure to phthalates influence thyroid function and growth hormone homeostasis? The Taiwan Environmental Survey for Toxicants (TEST) 2013. Environ Res 153:63–72
Hwang YH, Paik MJ, Yee ST (2017) Diisononyl phthalate induces asthma via modulation of Th1/Th2 equilibrium. Toxicol Lett 272:49–59
Ibhazehiebo K, Koibuchi N (2011) Thyroid hormone receptor-mediated transcription is suppressed by low dose phthalate. Niger J Physiol Sci 26:143–149
Ikegami K, Refetoff S, Van Cauter E, Yoshimura T (2019) Interconnection between circadian clocks and thyroid function. Nat Rev Endocrinol 15:590–600
Izumi M, Larsen PR (1977) Triiodothyronine, thyroxine, and iodine in purified thyroglobulin from patients with Graves’ disease. J Clin Invest 59:1105–1112
Jia PP, Ma YB, Lu CJ, Mirza Z, Zhang W, Jia YF et al (2016) The effects of disturbance on hypothalamus-pituitary-thyroid (HPT) axis in zebrafish larvae after exposure to DEHP. PLoS One 11:e0155762
Jiao F, Qiao K, Jiang Y, Li S, Zhao J, Gui W (2019) Integrated thyroid endocrine disrupting effect on zebrafish (Danio rario) larvae via simultaneously repressing type II iodothyronine deiodinase and activating thyroid receptor-mediated signaling following waterborne exposure to trace azocyclotin. Environ Pollut 255:113328
Johns LE, Ferguson KK, Soldin OP, Cantonwine DE, Rivera-Gonzalez LO, Del Toro LV et al (2015) Urinary phthalate metabolites in relation to maternal serum thyroid and sex hormone levels during pregnancy: a longitudinal analysis. Reprod Biol Endocrinol 13:4
Johns LE, Ferguson KK, McElrath TF, Mukherjee B, Meeker JD (2016) Associations between Repeated Measures of Maternal Urinary Phthalate Metabolites and Thyroid Hormone Parameters during Pregnancy. Environ Health Perspect 124:1808–1815
Kambia N, Séverin I, Farce A, Dahbi L, Dine T, Moreau E, et al. 2021 Comparative effects of di-(2-ethylhexyl)phthalate and di-(2-ethylhexyl)terephthalate Metabolites on thyroid receptors: in vitro and in silico studies. Metabolites; 11
Kamrin MA, Mayor GH (1991) Diethyl phthalate: a perspective. J Clin Pharmacol 31:484–489
Kang JC, Jee JH, Koo JG, Keum YH, Jo SG, Park KH (2010) Anti-oxidative status and hepatic enzymes following acute administration of diethyl phthalate in olive flounder Paralichthys olivaceus, a marine culture fish. Ecotoxicol Environ Saf 73:1449–1455
Kang J, Song J, Shen S, Li B, Yang X, Chen M (2016) Diisononyl phthalate aggravates allergic dermatitis by activation of NF-kB. Oncotarget 7:85472–85482
Kasper-Sonnenberg M, Koch HM, Apel P, Ruther M, Palmke C, Bruning T et al (2019) Time trend of exposure to the phthalate plasticizer substitute DINCH in Germany from 1999 to 2017: Biomonitoring data on young adults from the Environmental Specimen Bank (ESB). Int J Hyg Environ Health 222:1084–1092
Kastner J, Cooper DG, Maric M, Dodd P, Yargeau V (2012) Aqueous leaching of di-2-ethylhexyl phthalate and “green” plasticizers from poly(vinyl chloride). Sci Total Environ 432:357–364
Kavlock R, Boekelheide K, Chapin R, Cunningham M, Faustman E, Foster P et al (2002) NTP center for the evaluation of risks to human reproduction: phthalates expert panel report on the reproductive and developmental toxicity of di(2-ethylhexyl) phthalate. Reprod Toxicol 16:529–653
Kim SM, Yoo JA, Baek JM, Cho KH (2015) Diethyl phthalate exposure is associated with embryonic toxicity, fatty liver changes, and hypolipidemia via impairment of lipoprotein functions. Toxicol in Vitro 30:383–393
Kim MJ, Moon S, Oh BC, Jung D, Choi K, Park YJ (2019) Association Between diethylhexyl phthalate exposure and thyroid function: a meta-analysis. Thyroid 29:183–192
Koch HM, Lorber M, Christensen KL, Palmke C, Koslitz S, Bruning T (2013) Identifying sources of phthalate exposure with human biomonitoring: results of a 48h fasting study with urine collection and personal activity patterns. Int J Hyg Environ Health 216:672–681
Kuo FC, Su SW, Wu CF, Huang MC, Shiea J, Chen BH et al (2015) Relationship of urinary phthalate metabolites with serum thyroid hormones in pregnant women and their newborns: a prospective birth cohort in Taiwan. PLoS One 10:e0123884
Lamb JC IV, Chapin RE, Teague J, Lawton AD, Reel JR (1987) Reproductive effects of four phthalic acid esters in the mouse. Toxicol Appl Pharmacol 88:255–269
Larsson K, Lindh CH, Jonsson BA, Giovanoulis G, Bibi M, Bottai M et al (2017) Phthalates, non-phthalate plasticizers and bisphenols in Swedish preschool dust in relation to children’s exposure. Environ Int 102:114–124
Lee YJ Lee E Kim TH Choi JS Lee J Jung KK et al. 2009 Effects of di (2-ethylhexyl) phthalate on regulation of steroidogenesis or spermatogenesis in testes of Sprague-Dawley rats.; 55: 380–388
Lima FR, Goncalves N, Gomes FC, de Freitas MS, Moura NV (1998) Thyroid hormone action on astroglial cells from distinct brain regions during development. Int J Dev Neurosci 16:19–27
Liu C, Zhao L, Wei L, Li L (2015) DEHP reduces thyroid hormones via interacting with hormone synthesis-related proteins, deiodinases, transthyretin, receptors, and hepatic enzymes in rats. Environ Sci Pollut Res Int 22:12711–12719
Liu C, Deng YL, Zheng TZ, Yang P, Jiang XQ, Liu EN et al (2020) Urinary biomarkers of phthalates exposure and risks of thyroid cancer and benign nodule. J Hazard Mater 383:121189
Magdouli S, Daghrir R, Brar SK, Drogui P, Tyagi RD (2013) Di 2-ethylhexylphtalate in the aquatic and terrestrial environment: a critical review. J Environ Manage 127:36–49
Manzano J, Bernal J, Morte B (2007) Influence of thyroid hormones on maturation of rat cerebellar astrocytes. Int J Dev Neurosci 25:171–179
Marotta V, Russo G, Gambardella C, Grasso M, La Sala D, Chiofalo MG et al (2019) Human exposure to bisphenol AF and diethylhexylphthalate increases susceptibility to develop differentiated thyroid cancer in patients with thyroid nodules. Chemosphere 218:885–894
Marotta V, Malandrino P, Russo M, Panariello I, Ionna F, Chiofalo MG et al (2020) Fathoming the link between anthropogenic chemical contamination and thyroid cancer. Crit Rev Oncol Hematol 150:102950
Martinez-Galan JR, Pedraza P, Santacana M, Escobar del Ray F, de MorrealeEscobar G, Ruiz-Marcos A (1997) Early effects of iodine deficiency on radial glial cells of the hippocampus of the rat fetus. A model of neurological cretinism. J Clin Invest 99:2701–9
Masutomi N, Shibutani M, Takagi H, Uneyama C, Takahashi N, Hirose M (2003) Impact of dietary exposure to methoxychlor, genistein, or diisononyl phthalate during the perinatal period on the development of the rat endocrine/reproductive systems in later life. Toxicology 192:149–170
Meeker JD, Ferguson KK (2011) Relationship between urinary phthalate and bisphenol A concentrations and serum thyroid measures in U.S. adults and adolescents from the National Health and Nutrition Examination Survey (NHANES) 2007–2008. Environ Health Perspect 119:1396–402
Meeker JD, Calafat AM, Hauser R (2007) Di(2-ethylhexyl) phthalate metabolites may alter thyroid hormone levels in men. Environ Health Perspect 115:1029–1034
Miao H, Liu X, Li J, Zhang L, Zhao Y, Liu S et al (2020) Associations of urinary phthalate metabolites with risk of papillary thyroid cancer. Chemosphere 241:125093
Minguez-Alarcon L, Souter I, Chiu YH, Williams PL, Ford JB, Ye X et al (2016) Urinary concentrations of cyclohexane-1,2-dicarboxylic acid monohydroxy isononyl ester, a metabolite of the non-phthalate plasticizer di(isononyl)cyclohexane-1,2-dicarboxylate (DINCH), and markers of ovarian response among women attending a fertility center. Environ Res 151:595–600
Mohammed ET, Hashem KS, Ahmed AE, Aly MT, Aleya L, Abdel-Daim MM (2020) Ginger extract ameliorates bisphenol A (BPA)-induced disruption in thyroid hormones synthesis and metabolism: Involvement of Nrf-2/HO-1 pathway. Sci Total Environ 703:134664
Mu X, Chen X, Liu J, Yuan L, Wang D, Qian L et al (2020) A multi-omics approach reveals molecular mechanisms by which phthalates induce cardiac defects in zebrafish (Danio rerio). Environ Pollut 265:113876
Mughal BB, Fini JB, Demeneix BA (2018) Thyroid-disrupting chemicals and brain development: an update. Endocr Connect 7:R160–R186
Mullur R, Liu YY, Brent GA (2014) Thyroid hormone regulation of metabolism. Physiol Rev 94:355–382
Nandi-Munshi D, Taplin CE (2015) Thyroid-related neurological disorders and complications in children. Pediatr Neurol 52:373–382
Nardelli TC, Albert O, Lalancette C, Culty M, Hales BF, Robaire B (2017) In Utero and Lactational Exposure Study in Rats to Identify Replacements for Di(2-ethylhexyl) Phthalate. Sci Rep 7:3862
Noda M (2018) Thyroid hormone in the CNS: contribution of neuron-glia interaction. Vitam Horm 106:313–331
Ortiz-Zarragoitia M, Trant JM, Cajaravillet MP (2006) Effects of dibutylphthalate and ethynylestradiol on liver peroxisomes, reproduction, and development of zebrafish (Danio rerio). Environ Toxicol Chem 25:2394–2404
Paluselli A, Fauvelle V, Schmidt N, Galgani F, Net S, Sempere R (2018) Distribution of phthalates in Marseille Bay (NW Mediterranean Sea). Sci Total Environ 621:578–587
Pan G, Hanaoka T, Yoshimura M, Zhang S, Wang P, Tsukino H et al (2006) Decreased serum free testosterone in workers exposed to high levels of di-n-butyl phthalate (DBP) and di-2-ethylhexyl phthalate (DEHP): a cross-sectional study in China. Environ Health Perspect 114:1643–1648
Park C, Choi W, Hwang M, Lee Y, Kim S, Yu S et al (2017) Associations between urinary phthalate metabolites and bisphenol A levels, and serum thyroid hormones among the Korean adult population - Korean National Environmental Health Survey (KoNEHS) 2012–2014. Sci Total Environ 584–585:950–957
Pecht MG, Ali I, Carlson A (2018) Phthalates in Electronics: The Risks and the Alternatives. Ieee Access 6:6232–6242
Peeters RP Visser TJ. Metabolism of Thyroid Hormone. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, et al., editors. Endotext, South Dartmouth (MA), 2000
Pollack GM, Li RC, Ermer JC, Shen DD (1985) Effects of route of administration and repetitive dosing on the disposition kinetics of di(2-ethylhexyl) phthalate and its mono-de-esterified metabolite in rats. Toxicol Appl Pharmacol 79:246–256
Revathy V, Chitra KJ (2018) Di-isononyl phthalate (DINP) impairs reproduction in the freshwater fish, Oreochromis mossambicus (Peters 1852). Asian Fish Sci 31:284–296
Roberts SC, Bianco AC, Stapleton HM (2015) Disruption of type 2 iodothyronine deiodinase activity in cultured human glial cells by polybrominated diphenyl ethers. Chem Res Toxicol 28:1265–1274
Romano ME, Eliot MN, Zoeller RT, Hoofnagle AN, Calafat AM, Karagas MR et al (2018) Maternal urinary phthalate metabolites during pregnancy and thyroid hormone concentrations in maternal and cord sera: The HOME Study. Int J Hyg Environ Health 221:623–631
Santangeli S, Maradonna F, Zanardini M, Notarstefano V, Gioacchini G, Forner-Piquer I et al (2017) Effects of diisononyl phthalate on Danio rerio reproduction. Environ Pollut 231:1051–1062
Schoonover CM, Seibel MM, Jolson DM, Stack MJ, Rahman RJ, Jones SA et al (2004) Thyroid hormone regulates oligodendrocyte accumulation in developing rat brain white matter tracts. Endocrinology 145:5013–5020
Schutze A, Kolossa-Gehring M, Apel P, Bruning T, Koch HM (2014) Entering markets and bodies: increasing levels of the novel plasticizer Hexamoll(R) DINCH(R) in 24 h urine samples from the German Environmental Specimen Bank. Int J Hyg Environ Health 217:421–426
Schwedler G, Conrad A, Rucic E, Koch HM, Leng G, Schulz C et al (2020) Hexamoll(R) DINCH and DPHP metabolites in urine of children and adolescents in Germany. Human biomonitoring results of the German Environmental Survey GerES V, 2014–2017. Int J Hyg Environ Health 229:113397
Serrano SE, Braun J, Trasande L, Dills R, Sathyanarayana S (2014) Phthalates and diet: a review of the food monitoring and epidemiology data. Environ Health 13:43
Sha Y, Xia X, Yang Z, Huang GH (2007) Distribution of PAEs in the middle and lower reaches of the Yellow River. China Environ Monit Assess 124:277–287
Shen OX, Du GZ, Sun H, Wu W, Jiang Y, Song L et al (2009) Comparison of in vitro hormone activities of selected phthalates using reporter gene assays. Toxicol Lett 191:9–14
Shen O, Wu W, Du G, Liu R, Yu L, Sun H et al (2011) Thyroid disruption by Di-n-butyl phthalate (DBP) and mono-n-butyl phthalate (MBP) in Xenopus laevis. PLoS One 6:e19159
Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP et al (2004) Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ Health Perspect 112(331):8
Sonde V, D’souza A, Tarapore R, Pereira L, Khare M, Sinkar P et al (2000) Simultaneous administration of diethylphthalate and ethyl alcohol and its toxicity in male Sprague-Dawley rats. Toxicology 147:23–31
Sugiyama S, Shimada N, Miyoshi H, Yamauchi K (2005) Detection of thyroid system-disrupting chemicals using in vitro and in vivo screening assays in Xenopus laevis. Toxicol Sci 88:367–374
Sun D, Zhou L, Wang S, Liu T, Zhu J, Jia Y et al (2018) Effect of Di-(2-ethylhexyl) phthalate on the hypothalamus-pituitary-thyroid axis in adolescent rat. Endocr J 65:261–268
Suzuki T, Yaguchi K, Suzuki S, Suga T (2001) Monitoring of phthalic acid monoesters in river water by solid-phase extraction and GC-MS determination. Environ Sci Technol 35:3757–3763
Tassinari R, Tait S, Busani L, Martinelli A, Narciso L, Valeri M et al (2021) Metabolic, reproductive and thyroid effects of bis(2-ethylhexyl) phthalate (DEHP) orally administered to male and female juvenile rats at dose levels derived from children biomonitoring study. Toxicology 449:152653
Tsai HJ, Wu CF, Tsai YC, Huang PC, Chen ML, Wang SL et al (2016) Intake of phthalate-tainted foods and serum thyroid hormones in Taiwanese children and adolescents. Sci Rep 6:30589
Tyler CR, Jobling S, Sumpter JP (1998) Endocrine disruption in wildlife: a critical review of the evidence. Crit Rev Toxicol 28:319–361
U.S.E.P.A (2020a) Agency, chemical data reporting (2012b and 2016 Public CDR database). Washington, DC: U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics. ChemView
U.S.E.P.A (2020b) Agency, Draft Use Report for Di-isononyl Phthalate (DINP) (1,2-Benzene-dicarboxylic acid, 1,2-diisononyl ester, and 1,2-Benzenedicarboxylic acid, di-C8–10-branched alkyl esters, C9-rich)
U.S.E.P.A (2012 ) Integrated risk information system
Vasconcelos AL, Silva MJ, Louro H (2019) In vitro exposure to the next-generation plasticizer diisononyl cyclohexane-1,2-dicarboxylate (DINCH): cytotoxicity and genotoxicity assessment in human cells. J Toxicol Environ Health A 82:526–536
Villanger GD, Drover SSM, Nethery RC, Thomsen C, Sakhi AK, Overgaard KR et al (2020) Associations between urine phthalate metabolites and thyroid function in pregnant women and the influence of iodine status. Environ Int 137:105509
Wang F, Xia X, Sha Y (2008) Distribution of phthalic acid esters in Wuhan section of the Yangtze River. China J Hazard Mater 154:317–324
Wang X, Wang L, Zhang J, Yin W, Hou J, Zhang Y et al (2018) Dose-response relationships between urinary phthalate metabolites and serum thyroid hormones among waste plastic recycling workers in China. Environ Res 165:63–70
Wang X, Yan X, Yang Y, Yang W, Zhang Y, Wang J et al (2020) Dibutyl phthalate-mediated oxidative stress induces splenic injury in mice and the attenuating effects of vitamin E and curcumin. Food Chem Toxicol 136:110955
Wei C, Ding S, You H, Zhang Y, Wang Y, Yang X et al (2011) An immunoassay for dibutyl phthalate based on direct hapten linkage to the polystyrene surface of microtiter plates. PLoS One 6:e29196
Weng TI, Chen MH, Lien GW, Chen PS, Lin JC, Fang CC, et al. 2017 Effects of Gender on the Association of Urinary Phthalate Metabolites with Thyroid Hormones in Children: A Prospective Cohort Study in Taiwan. Int J Environ Res Public Health; 14
Wenzel A, Franz C, Breous E, Loos U (2005) Modulation of iodide uptake by dialkyl phthalate plasticisers in FRTL-5 rat thyroid follicular cells. Mol Cell Endocrinol 244:63–71
WHO, 2011 Guidelines for drinking-water quality, WHO, World Health Organization
Williams GR (2008) Neurodevelopmental and neurophysiological actions of thyroid hormone. J Neuroendocrinol 20:784–794
Wu MT, Wu CF, Chen BH, Chen EK, Chen YL, Shiea J et al (2013) Intake of phthalate-tainted foods alters thyroid functions in Taiwanese children. PLoS One 8:e55005
Wu W, Zhou F, Wang Y, Ning Y, Yang JY, Zhou YK (2017) Exposure to phthalates in children aged 5–7years: Associations with thyroid function and insulin-like growth factors. Sci Total Environ 579:950–956
Wu Y, Li J, Yan B, Zhu Y, Liu X, Chen M et al (2017) Oral exposure to dibutyl phthalate exacerbates chronic lymphocytic thyroiditis through oxidative stress in female Wistar rats. Sci Rep 7:15469
Wu H Zhang W Zhang Y Kang Z Miao X Na X. 2021 Novel insights into di(2ethylhexyl)phthalate activation: Implications for the hypothalamuspituitarythyroid axis Mol Med Rep 23
Yang J, Hauser R, Goldman RH (2013) Taiwan food scandal: the illegal use of phthalates as a clouding agent and their contribution to maternal exposure. Food Chem Toxicol 58:362–368
Yao HY, Han Y, Gao H, Huang K, Ge X, Xu YY et al (2016) Maternal phthalate exposure during the first trimester and serum thyroid hormones in pregnant women and their newborns. Chemosphere 157:42–48
Zeng F, Cui K, Xie Z, Liu M, Li Y, Lin Y et al (2008) Occurrence of phthalate esters in water and sediment of urban lakes in a subtropical city, Guangzhou. South China Environ Int 34:372–380
Zhai W, Huang Z, Chen L, Feng C, Li B, Li T (2014) Thyroid endocrine disruption in zebrafish larvae after exposure to mono-(2-ethylhexyl) phthalate (MEHP). PLoS One 9:e92465
Zhang YH, Zheng LX, Chen BH (2006) Phthalate exposure and human semen quality in Shanghai: a cross-sectional study. Biomed Environ Sci 19:205–209
Zhang W, Shen XY, Zhang WW, Chen H, Xu WP, Wei W (2017) The effects of di 2-ethyl hexyl phthalate (DEHP) on cellular lipid accumulation in HepG2 cells and its potential mechanisms in the molecular level. Toxicol Mech Methods 27:245–252
Zoeller RT (2005) Environmental chemicals as thyroid hormone analogues: new studies indicate that thyroid hormone receptors are targets of industrial chemicals? Mol Cell Endocrinol 242:10–15
Acknowledgements
We thank Örebro University and granting agencies for supporting this study.
Funding
Open access funding provided by Örebro University. This study was financed by the Knowledge Foundation, Sweden; Helge Ax:son Johnsons Foundation; Längmanska Culture Foundation; Magnus Bergvalls Foundation; and Örebro University, Sweden.
Author information
Authors and Affiliations
Contributions
CB: manuscript writing and editing. AP: manuscript writing, editing, and funding acquisition.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Mohamed M. Abdel-Daim.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bereketoglu, C., Pradhan, A. Plasticizers: negative impacts on the thyroid hormone system. Environ Sci Pollut Res 29, 38912–38927 (2022). https://doi.org/10.1007/s11356-022-19594-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-19594-0