Abstract
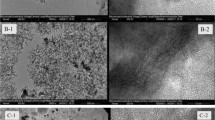
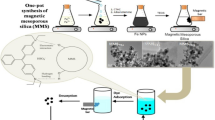
A facile soft-template solvent thermal strategy was developed to prepare mesoporous carbon–silica composite (MMCS) by using furfuryl alcohol (FA) as carbon precursor, Pluronic copolymer P123 as template, hydrated iron nitrate as iron source, and teraethylorthosilicate as silicon source and it was applied for the removal of methyl orange (MO). The as-synthesized MMCS with abound of hydrophilic groups processed a high specific surface area, large pore volume, and good magnetic response. With the increased amount of FA, the surface area and functional groups increased, promoting the adsorption effect. The maximum adsorption capacity of MO on MMCS can be high to 113.1 mg g−1 at pH 4 with 150 mg L−1 initial MO concentration. The adsorption isotherm, kinetic, and thermodynamics were all studied and the results showed the adsorption process well fitted Langmuir adsorption isotherm and pseudo-second-order model. Additionally, it was shown that the adsorption process could not be interfered by the co-existence of PO43−, NO3−, CO32−, SO42−, and real water matrix. And the proposed adsorbent can remove MO in three practical water samples with satisfied removal rates ranging from 92.8 to 99.8%. Thus, the MMCS prepared in this study could be utilized as an alternative adsorbent for the removal of methyl orange from practical aqueous solution.






Similar content being viewed by others
References
Arica TA, Ayas E, Arica MY (2017) Magnetic MCM-41 silica particles grafted with poly(glycidylmethacrylate) brush: modification and application for removal of direct dyes. Micropor Mesopor Mat 243:164–175
Cao Y, Wang YX, Zhang XY, Cai XG, Li ZH, Li GT 2019 Facile synthesis of 3D Mg-Al layered double oxide microspheres with ultra high adsorption capacity towards methyl orange. Mater Lett 257 126695
Chen RN, Zhai SM, Lu WH, Wei JW, Xu JY, Lu AM, Jiang HM (2020) Facile one-pot solvothermal synthesis of magnetic mesoporous carbon for the efficient adsorption of methyl orange. Environ Sci Pollut R 27:8248–8259
Chen SH, Zhang J, Zhang CL, Yue QY, Li Y, Li C (2010) Equilibrium and kinetic studies of methyl orange and methyl violet adsorption on activated carbon derived from Phragmites australis. Desalination 252(1–3):149–156
Deng J, Chen YJ, Lu YA, Ma XY, Feng SF, Gao NY, Li J (2017) Synthesis of magnetic CoFe2O4/ordered mesoporous carbon nanocomposites and application in Fenton-like oxidation of rhodamine B. Environ Sci Pollut Res 24:14396–14408
Deng YH, Cai Y, Sun ZK, Zhao DY (2011) Magnetically responsive ordered mesoporous materials: a burgeoning family of functional composite nanomaterials. Chem Phys Lett 510:1–13
Firouzi A, Kumar D, Bull LM, Besier T, Sieger P, Huo Q, Walker A, Zasadzinsky JA, Glinka C, Nieol J, Margolesse D, Stueky GD, Chmelka BF (1995) Cooperative organization of inorganic-surfactant and biomimetic assemblies. Science 267:1138–1143
Guo XY, Du B, Wei Q, Yang J, Hu LH, Yan LG, Xu WY (2014) Synthesis of amino functionalized magnetic graphenes composite material and its application to remove Cr(VI), Pb(II), Hg(II), Cd(II) and Ni(II) from contaminated water. J Hazard Mater 278:211–220
Hu X, Jia LJ, Cheng J, Sun ZR (2019) Magnetic ordered mesoporous carbon materials for adsorption of minocycline from aqueous solution: preparation, characterization and adsorption mechanism. J Hazard Mater 362:1–8
Jiang XC, Xiang XT, Peng SJ, Hou LX (2019) Facile preparation of nitrogen-doped activated mesoporous carbon aerogel from chitosan for methyl orange adsorption from aqueous solution. Cellulose 26:4515–4527
Liang T, Wang FX, Liang L, Liu MS, Sun JM (2016) Magnetically separable nitrogen-doped mesoporous carbon with high adsorption capacity. J Mater Sci 51:3868–3879
Liu H, Luo SH, Hu DB, Liu X, Wang Q, Wang ZY, Wang YL, Chang LJ, Liu YG, Yi TF, Zhang YH, Hao AM 2019 Design and synthesis of carbon-coated α-Fe2O3@Fe3O4 heterostructured as anode materials for lithium ion batteries. Appl. Surf. Sci. 495 143590
Liu YY, Zeng GM, Tang L, Cai Y, Pang Y, Zhang Y, Yang GD, Zhou YY, He XX, He Y (2015) Highly effective adsorption of cationic and anionic dyes on magnetic Fe/Ni nanoparticles doped bimodal mesoporous carbon. J Colloid Interf Sci 448:451–459
Ma CF, Gao Q, Zhou J, Chen QX, Han B, Xia KS, Zhou CG (2017) Facile one-pot synthesis of magnetic nitrogendoped porous carbon for high-performance bilirubin removal from BSA-rich solution. RSC Adv 7:2081–2091
Ma J, Yu F, Zhou L, Jin L, Yang MX, Luan JS, Tang YH, Fan HB, Yuan ZW, Chen JH (2012) Enhanced adsorption removal of methyl orange and methylene blue from aqueous solution by alkali-activated multiwalled carbon nanotubes. ACS Appl Mater Interfaces 4:5749–5760
Nejad NF, Shams E, Amini MK, Bennett JC (2013) Synthesis of magnetic mesoporous carbon and its application for adsorption of dibenzothiophene. Fuel Process Technol 106:376–384
Peng C, Dai J, Yu JY, Yin J (2015) Calcined Mg-Fe layered double hydroxide as an absorber for the removal of methyl orange. AIP Adv 5:57138
Peng XM, Hu XJ, Fu DF, Lam FLY (2014) Adsorption removal of acid black 1 from aqueous solution using ordered mesoporous carbon. Appl Surf Sci 294:71–80
Peng SY, Mao TY, Zheng C, Wu X, Wei Y, Zeng ZW, Xiao RH, Sun Y 2019 Polyhydroxyl gemini surfactant-modified montmorillonite for efficient removal of methyl orange. Colloid Surface A 578: 123602.
Phenrat T, Liu YQ, Tilton RD, Lowry GV (2009) Adsorbed polyelectrolyte coatings decrease Fe0 nanoparticle reactivity with TCE in water: conceptual model and mechanisms. Environ Sci Technol 43:1507–1514
Rashid RA, Jawad AH, Ishak MAM, Kasim NN (2016) KOH-activated carbon developed from biomass waste: adsorption equilibrium, kinetic and thermodynamic studies for methylene blue uptake. Desal Water Treat 57(56):27226–27236
Saroyan HS, Giannakoudakis DA, Sarafidis CS, Lazaridis NK, Deliyanni EA (2017) Effective impregnation for the preparation of magnetic mesoporous carbon: application to dye adsorption. J Chem Technol Biot 92(8):1899–1911
Schneider M, Ballweg T, Groß L, Gellermann C, Sanchez-Sanchez A, Fierro V, Celzard A, Mandel K (2019) Magnetic carbon composite particles for dye adsorption from water and their electrochemical regeneration. Part Part Syst Charact 36:1800537
Tang L, Yang GD, Zeng GM, Cai Y, Li SS, Zhou YY, Pang Y, Liu YY, Zhang Y, Luna B (2014a) Synergistic effect of iron doped ordered mesoporous carbon on adsorption-coupled reduction of hexavalent chromium and the relative mechanism study. Chem Eng J 239:114–122
Tang L, Cai Y, Yang G, Liu YY, Zeng GM, Zhou YY, Li SS, Wang JJ, Zhang S, Fang Y, He YB (2014b) Cobalt nanoparticles-embedded magnetic ordered mesoporous carbon for highly effective adsorption of rhodamine B. Appl Surf Sci 314:746–753
Tang L, Xie ZH, Zeng GM, Dong HR, Fan CZ, Zhou YY, Wang JJ, Deng YC, Wang JJ, Wei X (2016) Removal of bisphenol A by iron nanoparticles doped magnetic ordered mesoporous carbon. RSC Adv 6(31):25724–25732
Wang XF, Huang AL, Zhong SN, Pan YF, Tian Y (2015a) Facile preparation of mesoporous carbon microspheres containing nickel nanoparticle and dye adsorption behavior. Sci Adv Mater 7:43–49
Wang YG, Yao MC, Chen YT, Zuo YH, Zhang XD, Cui LF (2015b) General synthesis of magnetic mesoporous FeNi/graphitic carbon nanocomposites and their application for dye adsorption. J Alloy Compo 627:7–12
Wu Q, Li W, Wu YJ, Zong GG, Liu SX (2015) Effect of reaction time on structure of ordered mesoporous carbon microspheres prepared from carboxymethyl cellulose by soft-template method. Ind Crop Prod 76:866–872
Wu ZX, Li W, Webley PA, Zhao DY (2012) General and controllable synthesis of novel mesoporous magnetic iron Oxide@Carbon encapsulates for efficient arsenic removal. Adv Mater 24:485–491
Yan TT, Chen H, Jiang F, Wang X (2014) Adsorption of perfluorooctane sulfonate and perfluorooctanoic acid on magnetic mesoporous carbon Nitride. J Chem Eng 59:508–515
Yuso AM, Meins JL, Oumellal Y, Paul-Boncour V, Zlotea C, Ghimbeu CM (2016) Facile and rapid one-pot microwave-assisted synthesis of Pd-Ni magnetic nanoalloys confined in mesoporous carbons. J Nanopart Res 18:380
Zhai YP, Dou YQ, Liu XX, Tu B, Zhao DY (2009) One-pot synthesis of magnetically separable ordered mesoporous carbon. J Mater Chem 19:3292–3300
Zhang L, Xiong Z, Chen Y, Peng L, Yu B, Gao X, Zhang R, Zhang L, Zhang W (2016) Soft-template synthesis of hydrophilic metallic zirconia nanoparticle-incorporated ordered mesoporous carbon composite and its application in phosphopeptide enrichment. RSC Adv 6:30014–30020
Zhang L, Miu WB, Yao JL, Sun L, Yu BH (2018) Magnetic ordered mesoporous carbon composites incorporating Ag nanoparticles as SERS substrate for enrichment and detection of trace mercaptan compounds. Res Chem Intermed 44:3365–3374
Zhang MM, Yu ZH, Yu HC (2020) Adsorption of eosin Y, methyl orange and brilliant green from aqueous solution using ferroferric oxide/polypyrrole magnetic composite. Polym Bull 77:1049–1066
Zhu XJ, Chong J, Hu T, Wang XF, Tian Y (2017) Enhanced stability and metallic modification of polymeric and carbonaceous nanospheres through precursor engineering via a one-pot aqueous strategy assisted by iron ions. J Mater Chem a 5:8297–8306
Funding
This work was supported by the National Natural Science Foundation of China (21607075) and Fundamental Research Funds for the Central Universities (KJQN201721 and KYZ201600163).
This research does not involve human participants and/or animals.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by BZ and JX. The first draft of the manuscript was written by BZ and the manuscript was revised by JX. Some supplementary experiments were carried out by ZX. And all the work was under the supervision of MW and HJ. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval
We approve and agree all the ethical guidelines proposed by the journal and make sure to respect third parties rights such as copyright and/or moral rights.
Consent for publication
All the authors content to publish this manuscript.
Consent to participate
All the authors content to participate in this research work.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Tito Roberto Cadaval Jr.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhu, B., Xu, J., Xu, Z. et al. Soft-template solvent thermal method synthesis of magnetic mesoporous carbon–silica composite for adsorption of methyl orange from aqueous solution. Environ Sci Pollut Res 29, 40734–40744 (2022). https://doi.org/10.1007/s11356-021-18135-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-18135-5