Abstract
The paper presents the photodegradation process of one-, two- and three-component dye mixtures by ZnO-SnO2 nanoparticles. After 60 min of running the processes, the dye removal efficiencies of 76.44, 72.69, 62.43, 77.00 and 92.46% for MB, RB, TB, MO and YQ degradation, respectively, were obtained. For binary and ternary systems, dye removal efficiencies for all cases exceeded 70%. When the binary and ternary dye mixtures were tested, the photodegradation efficiencies of ZnO-SnO2 were similar to those of the single mixtures, indicating that this material could be used in industrial applications in the future. The focus of the study was to investigate the effect of sorption on photodegradation efficiency and the presence of both cationic and anionic dyes on their degradation efficiency under UV light. The significance of the effect of sorption on the degradation efficiency allowing the interaction of the catalyst with the dyes removed was confirmed. The main factor influencing sorption and consequently photocatalysis was the nature of the dye. It was confirmed that the positively charged ZnO-SnO2 surface effectively sorbs the dyes and causes their degradation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to the increase of pollutants that are present in water, the use of standard water treatment methods is insufficient. Therefore, new pollutant removal technologies that are effective, economical and environmentally friendly are being intensively sought. One such method is the removal of contaminants from the aqueous phase by photocatalytic degradation of organic compounds in the presence of ZnO nanostructures (Jiang and Pi 2018). The nanoscale zinc oxide can currently be considered one of the most important semiconducting oxides. This is due to its unique properties, including high chemical stability, high electrochemical coupling coefficient, wide radiation absorption range and high photostability (Siddiqi et al. 2018). Zinc oxide has a wider, compared to TiO2, energy band which is about 3.3 eV, high binding energy (60 meV) and high thermal and mechanical stability (Promnopas et al. 2015). Nanoparticles and nanocomposites used for water and wastewater treatment are gaining popularity. Apart from TiO2, zinc(II) oxide is one of the most commonly reported semiconductors used in photodegradation processes. Modification of ZnO can be achieved by addition of SnO2, α-Fe2O3, WO3, ZrO2 (Davari et al. 2017; Sorbiun et al. 2018; Ebrahimi et al. 2019; Qi et al. 2020). Two important semiconductors, ZnO and SnO2, have attracted considerable attention due to their unique conduction properties, mechanical properties, simple microstructure and corresponding conduction and conduction band gap (Karpuraranjith and Thambidurai 2017a; Isabel Bento Rovisco et al. 2020).
Complex structures of photocatalysts are obtained using various methods (Enesca and Andronic 2021). The literature reports, among others, the use of TiO2 to form a heterogeneous structure with LaNiO3 perovskite by sol–gel method and confirmation of the photocatalytic activity of the obtained material (Zhang and Jaroniec 2018; Chen et al. 2020). The fabrication of two- (CuxS/SnO2) and multicomponent (TiO2/CuxS-CuO/SnO2 and ZnO/CuxS-CuO/SnO2) thin film tandem structures by robotic spray pyrolysis representing a potential material for UV–Vis degradation of pollutants was also described (Enesca et al. 2015). The co-precipitation method produced a BiOBr/BiORBr-x heterojunction with excellent photocatalytic activity for degradation of many contaminants under visible light (Li et al. 2020). An s-scheme BiOBr/BiOAc1-xBrx heterojunction was also formed at room temperature in which the lifetime of the carriers with stronger redox capability was extended (Jia et al. 2020).
In contrast to the passive removal of pollutants that takes place in filtration processes, sorption, etc., photocatalysis degrades organic pollutants in water or wastewater by oxidation of free radicals and reduction of hydration electrons (Atchudan et al. 2017; Zhang and Zhang 2020). Photocatalysis using solid-state oxide materials is a widespread technique used to remove a wide variety of organic contaminants (Kashinath et al. 2017b). Recently, research has been conducted on advanced oxidation methods. Such methods include heterogeneous solid-state photocatalysis (Ayodhya and Veerabhadram 2018). It enables the efficient generation of hydroxyl radicals for the complete removal of organic contaminants, mainly of pharmaceutical origin. Recent studies have shown that heterogeneous photocatalysis is the most efficient technique for the degradation of dyes (Kashinath et al. 2017a; Chiu et al. 2019; Sun et al. 2020). This method made it possible to completely decompose organic compounds into harmless substances such as CO2 and H2O.
Much of the research on the photodegradation of organic compounds is based on the analysis of single-component systems. Mixtures of dyes, active compounds, drugs, etc. may have different effects than individual forms and may increase or decrease toxicity (Arshad et al. 2018; Medhi et al. 2019). In industrial wastewater treatment processes, including photocatalytic processes, there are various parameters that affect the treatment efficiency. These parameters include catalyst mass, dye concentration, pH, light intensity, photocatalyst type and temperature. They influence the rate of decomposition of individual components, the form of their occurrence and the activity of the photocatalyst itself. Additional parameters omitted from the analysis are the presence of additional compounds, the analysis of interactions between individual components and between the components and the catalyst surface (Rovisco et al. 2021).
Among the organic pollutants found in wastewater, dyes are an important group. Dyes are widely used in various branches of industry. They can be found, for example, in the textile, tanning and fur, paper and wood industries, construction and furniture, household and automotive chemical industries. Dyes are contaminants which are difficult to remove during the treatment process. Dyes may be highly toxic to aquatic organisms and may interfere with certain chemical and biological processes such as photosynthesis and oxygen solubility (Karpuraranjith and Thambidurai 2017b; Saeidi et al. 2019). A significant proportion of dyes belong to the group of azo dyes. Due to their complex chemical structure, the molecules are resistant to light, oxidising conditions and decomposition by microorganisms.
The aim of the study is to indicate the possibility of using ZnO nanoparticles modified with tin(IV) oxide as a photocatalyst in the processes of decomposition of dyes in aqueous solution under UV light in single and multicomponent systems. The novelty in the paper includes an attempt to explain the mechanism of interaction between sorption and photodegradation processes and the analysis of dye decomposition from multicomponent solutions, i.e. solutions that are actually found in wastewater. In this way, it is possible to better describe the removal processes of organic pollutants and maximise their removal efficiency by combining the sorption and photocatalytic properties of the material used for their removal. The research carried out in this work is a response to the current problems of pollution of the aqueous environment by dyes. The obtained system was tested for photocatalytic properties in the processes of dye decomposition in aqueous solution under UV light by analysing the interactions between the individual components present in the systems.
Materials and methods
Materials
For the preparation of modified zinc oxide (ZnO NPs), the following reagents were used: ZnSO4∙7H2O (98%, POCH), SnCl4 (> 99%, Sigma Aldrich) and Na2CO3 (> 99%, Sigma Aldrich). The photocatalytic properties of the materials were verified by dye degradation processes (Table 1) The dyes were selected due to the difference in particle size (molar masses ranging from 327.2 to 872.9 g/mol) and the ionic nature of the dyes (anionic, cationic and neutral). The dyes were from Sigma-Aldrich. Solutions were prepared by dissolving the compounds in deionised water.
Methodology
In order to obtain ZnO-SnO2 NPs, zinc oxide was obtained as the particle core in the first step. For this purpose, 10 ml of 2 M Na2CO3 solution was added to 20 ml of 1 M ZnSO4 salt solution in the presence of ultrasound. The precipitated metal hydroxides were dehydrated in a microwave reactor at 180 °C to obtain ZnO NPs. The material was filtered, washed with water, and dried at 105 °C. In the second step, 0.768 g of ZnO NPs was weighed, and a solution of SnCl4 was added in a volume of 20 ml 1 M such that SnO2 constituted 10%mas in the final product. A 2 M solution of Na2CO3 was added to the suspension in the presence of ultrasound in a stoichiometric ratio. The precipitated Sn(OH)4 hydroxide deposited on ZnO oxide was dehydrated in a microwave reactor at 180 °C to obtain ZnO-SnO2 nanoparticles. The material was filtered, washed with water, and dried at 105 °C.
Photodegradation of dyes in systems
The study of the photodegradation of dyes in the presence of a photocatalyst was carried out by adding 200 mg of catalyst to 60 ml of 18 mg/dm3 dye solution. After 60 min of sorption in the absence of light, the samples were exposed to 365 nm ultraviolet radiation (UV light). The lamps were placed 20 cm above the samples. The entire system was protected to prevent exposure to other light sources. The calculated value of the light irradiance was 5.3 mW/cm2. During the study of photodegradation processes of binary systems, the concentration of dyes was 9 mg/dm3 each, and the concentration of dyes in ternary systems was 6 mg/dm3 each, so that the total initial concentration of dyes of the system was constantly 18 mg/dm3.
The efficiency of dye removal from solution in single-component systems by photodegradation was determined from the relationship:
where \(E\) is the decomposition efficiency of the selected dye and \({C}_{0}\) is the initial concentration of the dye. For single-component systems, the amount of dye removed from solution per photocatalyst was also determined:
where \(V\) is the volume of the dye solution (ml) and \(m\) is the mass of the photocatalyst (g). In multicomponent systems, overlapping of spectra from individual dyes is observed, resulting in a mixture spectrum.
Methods
The phase composition of the materials obtained was investigated by XRD (Philips X’Pert camera with monochromator PW 1752/00 CuKα). The structure of ZnO-SnO2 was investigated by scanning electron microscopy (SEM) with EDS to obtain a mapping of the surface composition of the material. The diffuse reflectance spectroscopy was performed in the range of 200–700 nm. Reflectance spectra were recorded using a UV-2600 spectrophotometer (Shimadzu). The concentration of dyes was determined by spectrophotometry (Rayleigh UV-1800 spectrophotometer). Calibration curves were prepared with the absorbance characteristics of each dye.
The value of zero-point exchange was examined in using dynamic light scattering (DLS) (Malvern Instruments, ZS-90 and Brookhaven, ZetaPALS). To 50 ml H2O, 0.1 M NaOH/HCl was added to establish pH in the range of 2 to 12. Such prepared solution (50 ml) was mixed with 25 mg ZnO-SnO2 nanoparticles. Suspensions were mixed by 24 h and after the zeta potential in each pH were determined.
Research results and discussion
Characteristics of ZnO-SnO2 material
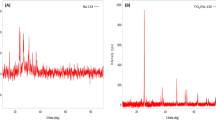
Modification of the surface of ZnO NPs with tin(IV) oxide caused increase of its photocatalytic properties. On the basis of XRD analysis, the superstructure of zinc oxide on ZnO and the formation of a new 2ZnO∙SnO2 phase can be observed (Fig. 1). The forming crystalline phase provided a change in the energy gap of the material and thus caused efficient electron transfer between the conduction band and the valence band.
Figure 2 presents SEM microphotographs with EDS analysis of ZnO-SnO2 NPs. The analysis revealed the preparation of ZnO rods and a 2ZnO∙SnO2 phase characterised by a non-formed particle shape and a particle size of about 1–5 μm.
The reflection spectrum of ZnO-SnO2 is shown in Fig. 3a. Based on the dependence of the Kubelka–Munk function, the width of the excited band was determined, which was 3.22 eV. The surface character of the photocatalyst was determined from the determined zero charge point (pzc) (Fig. 3b). If the pH of a suspended solid is above zero charge, it means that the surface is negatively charged and has the capacity to adsorb cations and participate in the cation exchange reaction (Fatehah et al. 2014), whereas when the pH is less than pzc, the surface is positively charged and has the ability to exchange anions. Processes carried out in neutral pH solutions result in the material having an affinity for neutral compounds and for anionic compounds. The photodegradation processes were carried out at neutral pH, hence a higher degree of degradation of the negative dyes: quinoline yellow and trypan blue were obtained.
Analysis of the photodegradation of dyes in the presence of ZnO-SnO2 NPs
To verify the photocatalytic activity of ZnO-SnO2, dyes differing in particle size, charge and structure were selected, i.e. methyl orange and trypan blue belonging to azo dyes, methylene blue belonging to azin dyes, rhodamine B as an example of a fluorescent dye and quinoline yellow a food dye. Under real conditions, a number of compounds are simultaneously present in the wastewater, making it necessary to take into account the interactions occurring between the compounds, which affect the overall removal efficiency of the system.
In the first step, the removal efficiencies of dyes from single-component systems were checked. Table 2 summarises the removal efficiencies of individual dyes after 60 min of photodegradation.
Among the dyes tested, the highest dye removal efficiency after photocatalysis was obtained for the degradation process of quinoline yellow, which value was 92.46% (Figs. 4 and 5). This is due to the fact that QY shows the lowest molecular weight, which resulted in increased adsorption of the dye on the catalyst surface (48.89%). Dye sorption is a key step in dye removal processes due to the surface nature of the photodegradation process. Other dyes were removed at a lower rate, but for all materials, the removal efficiency was above 60%. The reduced removal efficiency of TB is due to the increased initial concentration of the dye.
In the second stage, the efficiency of dye removal was verified in binary and ternary systems (Table 3).
In order to enable the photodegradation of compounds on the surface of the photocatalyst, it is necessary, at the first stage, to adsorb these compounds. The significance of sorption efficiency on the enhancement of photodegradation process was confirmed by analysis of variance (ANOVA) (Table 4).
Based on the results obtained, 2 areas of different influence of sorption on the photodegradation of dyes can be observed. In systems where the sorption efficiency does not exceed, 5% of the degree of degradation of dyes is about 70%. In subsequent systems, increasing the sorption efficiency results in a gradual decrease in the photocatalysis effect, which is due to a decrease in the sample concentration in solution. However, if the sorption share is 20–30%, the influence of the photodegradation effect increases again. Two different mechanisms can be observed here. On the one hand, an increase of the sorption effect results in a decrease of the total dye concentration that can be removed by photodegradation. The opposite effect is a favourable correlation due to the surface nature of the photodegradation process. An increase in the number of sorbed pigment particles increases the efficiency of the photodegradation process, which confirms the following phenomena (Lee et al. 2019; Rodwihok et al. 2021). Based on the results obtained, it can be concluded that it is advantageous for the photocatalytic material to be characterised by sorption properties, but the process should be physical in nature, so that photodegradation can take place and decomposition residues can be removed from the material surface.
Analysis of the influence of parameters on the sorption efficiency showed a significant effect of the amount of components in the system, the influence of the type and nature of the dye to be removed (Fig. 6). While keeping the concentration of dyes in the system constant, increasing the amount of components favourably affects their sorption on the material surface. Dyes of different structures gradually fill the surface of the catalyst, which makes it possible to obtain a higher degree of their uptake from the solution. The presence of other dyes in the system does not significantly impair the sorption properties. Based on the determined isoelectric point, the cationic character of the catalyst surface was confirmed. Due to the positively charged surface, higher sorption efficiency was observed for the anionic and neutral compounds, while significantly lower sorption efficiency was observed for the cationic dyes (Jose et al. 2021). In the case of the photodegradation process, an opposite relationship was observed. The cationic dyes are more easily separated from the catalyst surface, and the removal of the degradation residues from the positively charged surface is facilitated.
Analysis of the kinetics of dye degradation in the presence of a photocatalyst
The decomposition rates of dyes in aqueous solutions in the presence of a photocatalyst were compared with the kinetics of photodegradation processes proposed by Langmuir–Hinshelwood (L–H):
where \(k\) is the photodegradation rate constant \((mi{n}^{-1}\)) and \({C}_{0}\) is the initial dye concentration.
For the material with the highest photodegradation efficiency, i.e. ZnO-SnO2, the kinetics of decomposition of dyes, i.e. one-, two- and three-component mixtures, was studied. For this purpose, 2 ml of the suspension was taken from the system every 15 min, swabbed and analysed by spectrophotometry in the range from 300 to 1000 nm. The results are presented in Table 5. The decomposition rate constants of the dyes for single-component systems ranged from 0.0122 to 0.0295 min−1. In multicomponent systems, a decrease in the decomposition rate constant for quinoline yellow was observed. This is due to the blocking of the photocatalyst surface by the other components with higher molecular weight, limiting the sorption of YQ.
Conclusion
In the paper, the decomposition analysis of dyes from mono-, bi- and tri-component systems using ZnO-SnO2 NPs as photocatalyst has been reported. After 60 min of processes, dye removal efficiencies of 76.44, 72.69, 62.43, 77.00 and 92.46% were obtained for the degradation of methylene blue, rhodamine B, trypan blue, methyl orange and quinoline yellow, respectively. For binary and ternary systems, dye removal efficiencies for all processes exceeded 70%. The presence of components of the same nature did not cause a decrease in the decomposition rate of the dyes. Significant differences were observed for compounds differing in particle size, which may be due to blocking of surface access and competition of the components for the catalyst active sites. The removal efficiency of cationic dyes in the presence of neutral dyes and anionic dyes significantly decreases due to charge imbalance on the material surface.
Availability of data and materials
All the data and tools/models used for this work are publicly available.
References
Arshad M, Qayyum A, Abbas G et al (2018) Influence of different solvents on portrayal and photocatalytic activity of tin-doped zinc oxide nanoparticles. J Mol Liq 260:272–278. https://doi.org/10.1016/j.molliq.2018.03.074
Atchudan R, Edison TNJI, Perumal S et al (2017) Direct solvothermal synthesis of zinc oxide nanoparticle decorated graphene oxide nanocomposite for efficient photodegradation of azo-dyes. J Photochem Photobiol A Chem 337:100–111. https://doi.org/10.1016/j.jphotochem.2017.01.021
Ayodhya D, Veerabhadram G (2018) A review on recent advances in photodegradation of dyes using doped and heterojunction based semiconductor metal sulfide nanostructures for environmental protection. Mater Today Energy 9:83–113
Chen C, Zhou J, Geng J, et al (2020) Perovskite LaNiO3/TiO2 step-scheme heterojunction with enhanced photocatalytic activity. Applied Surface Science 503:144287. https://doi.org/10.1016/j.apsusc.2019.144287
Chiu Y-H, Chang T-F, Chen C-Y et al (2019) Mechanistic insights into photodegradation of organic dyes using heterostructure photocatalysts. Catalysts 9:430. https://doi.org/10.3390/catal9050430
Davari N, Farhadian M, Nazar ARS, Homayoonfal M (2017) Degradation of diphenhydramine by the photocatalysts of ZnO/Fe2O3 and TiO2/Fe2O3 based on clinoptilolite: structural and operational comparison. J Environ Chem Eng 5:5707–5720. https://doi.org/10.1016/j.jece.2017.10.052
Ebrahimi R, Maleki A, Zandsalimi Y et al (2019) Photocatalytic degradation of organic dyes using WO 3-doped ZnO nanoparticles fixed on a glass surface in aqueous solution. J Ind Eng Chem 73:297–305. https://doi.org/10.1016/j.jiec.2019.01.041
Enesca A, Andronic L (2021) Photocatalytic Activity of S-Scheme Heterostructure for Hydrogen Production and Organic Pollutant Removal: A Mini-Review. Nanomaterials 11:871–890. https://doi.org/10.3390/nano11040871
Enesca A, Isac L, Duta A (2015) Charge carriers injection in tandem semiconductors for dyes mineralization. Applied Catalysis B: Environmental 162:352–363. https://doi.org/10.1016/j.apcatb.2014.06.059
Fatehah MO, Aziz HA, Stoll S (2014) Stability of ZnO nanoparticles in solution. Influence of pH, Dissolution, Aggregation and Disaggregation Effects. J Colloid Sci Biotechnol 3:75–84. https://doi.org/10.1166/jcsb.2014.1072
Isabel Bento Rovisco A, Branquinho R, Vaz Pinto J, et al (2020) Hydrothermal synthesis of zinc tin oxide nanostructures for photocatalysis, energy harvesting and electronics. In: Novel Nanomaterials [Working Title]. IntechOpen
Jiang J, Pi J, Cai J (2018) The advancing of zinc oxide nanoparticles for biomedical applications. Bioinorg Chem Appl 2018
Jia X, Han Q, Liu H et al (2020) A dual strategy to construct flowerlike S-scheme BiOBr/BiOAc1−xBrx heterojunction with enhanced visible-light photocatalytic activity. Chemical Engineering Journal 399:125701. https://doi.org/10.1016/j.cej.2020.125701
Jose LM, Raj RSA, Sajan D, Aravind A (2021) Adsorption and photocatalytic activity of biosynthesised ZnO nanoparticles using Aloe vera leaf extract. Nano Express 2:010039. https://doi.org/10.1088/2632-959x/abeec6
Karpuraranjith M, Thambidurai S (2017a) Synergistic effect of chitosan-zinc-tin oxide colloidal nanoparticle and their binding performance on bovine albumin serum. Mater Chem Phys 199:370–378. https://doi.org/10.1016/j.matchemphys.2017.07.019
Karpuraranjith M, Thambidurai S (2017b) Hybrid structure of biotemplate-zinc-tin oxide for better optical, morphological and photocatalytic properties. Semicond Sci Technol 32:035014. https://doi.org/10.1088/1361-6641/aa577c
Kashinath L, Namratha K, Byrappa K (2017a) Sol-gel assisted hydrothermal synthesis and characterization of hybrid ZnS-RGO nanocomposite for efficient photodegradation of dyes. J Alloys Compd 695:799–809. https://doi.org/10.1016/j.jallcom.2016.10.063
Kashinath L, Namratha K, Srikantaswamy S et al (2017b) Microwave treated sol-gel synthesis and characterization of hybrid ZnS-RGO composites for efficient photodegradation of dyes. New J Chem 41:1723–1735. https://doi.org/10.1039/c6nj03716j
Lee Y, Lee S, Kim HS et al (2019) Multifunctional and recyclable TiO 2 hybrid sponges for efficient sorption, detection, and photocatalytic decomposition of organic pollutants. J Ind Eng Chem 73:328–335. https://doi.org/10.1016/j.jiec.2019.02.001
Li S, Han Q, Jia X, et al (2020) Room-temperature one-step synthesis of tube-like S scheme BiOBr/BiO(HCOO)Brx heterojunction with excellent visible-light photocatalytic performance. Applied Surface Science 530:147208. https://doi.org/10.1016/j.apsusc.2020.147208
Medhi R, Li CH, Lee SH et al (2019) Uniformly spherical and monodisperse antimony- and zinc-doped tin oxide nanoparticles for optical and electronic applications. ACS Appl Nano Mater 2:6554–6564. https://doi.org/10.1021/acsanm.9b01474
Promnopas W, Thongtem T, Thongtem S (2015) Effect of microwave power on energy gap of ZnO nanoparticles synthesized by microwaving through aqueous solutions. Superlattices Microstruct 78:71–78. https://doi.org/10.1016/j.spmi.2014.11.021
Qi K, Xing X, Zada A et al (2020) Transition metal doped ZnO nanoparticles with enhanced photocatalytic and antibacterial performances: experimental and DFT studies. Ceram Int 46:1494–1502. https://doi.org/10.1016/j.ceramint.2019.09.116
Rodwihok C, Charoensri K, Wongratanaphisan D et al (2021) Improved photocatalytic activity of surface charge functionalized ZnO nanoparticles using aniline. J Mater Sci Technol 76:1–10. https://doi.org/10.1016/j.jmst.2020.09.041
Rovisco A, Branquinho R, Deuermeier J et al (2021) Shape effect of zinc-tin oxide nanostructures on photodegradation of methylene blue and rhodamine B under UV and visible light. ACS Appl Nano Mater. https://doi.org/10.1021/acsanm.0c02782
Saeidi M, Abrari M, Ahmadi M (2019) Fabrication of dye-sensitized solar cell based on mixed tin and zinc oxide nanoparticles. Appl Phys A Mater Sci Process 125:409. https://doi.org/10.1007/s00339-019-2697-3
Siddiqi KS, ur Rahman A, Tajuddin, Husen A (2018) Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res Lett 13:1–13
Sorbiun M, ShayeganMehr E, Ramazani A, TaghaviFardood S (2018) Green synthesis of zinc oxide and copper oxide nanoparticles using aqueous extract of oak fruit hull (Jaft) and comparing their photocatalytic degradation of basic violet 3. Int J Environ Res 12:29–37. https://doi.org/10.1007/s41742-018-0064-4
Sun X, Ji S, Wang M et al (2020) Fabrication of porous TiO2-RGO hybrid aerogel for high-efficiency, visible-light photodegradation of dyes. J Alloys Compd 819:153033. https://doi.org/10.1016/j.jallcom.2019.153033
Zhang G, Zhang S (2020) Quantitative structure-activity relationship in the photodegradation of azo dyes. J Environ Sci (china) 90:41–50. https://doi.org/10.1016/j.jes.2019.11.009
Zhang L, Jaroniec M (2018) Toward designing semiconductor-semiconductor heterojunctions for photocatalytic applications. Applied Surface Science 430:2–17. https://doi.org/10.1016/j.apsusc.2017.07.192
Author information
Authors and Affiliations
Contributions
Conceptualisation, Olga Długosz; methodology, Paulina Brzoza and Anita Staroń; formal analysis and investigation, Paulina Brzoza and Anita Staroń; writing-original draft preparation, Olga Długosz; writing-review and editing, Marcin Banach; funding acquisition, Marcin Banach; resources, Marcin Banach; and supervision, Marcin Banach.
Corresponding author
Ethics declarations
Ethics approval
Not applicable. This manuscript does not involve researching about humans or animals.
Consent to participate
All of the authors consented to participate in the drafting of this manuscript.
Consent for publication
All of the authors consented to publish this manuscript.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible editor: Sami Rtimi
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Długosz, O., Staroń, A., Brzoza, P. et al. Synergistic effect of sorption and photocatalysis on the degree of dye removal in single and multicomponent systems on ZnO-SnO2. Environ Sci Pollut Res 29, 27042–27050 (2022). https://doi.org/10.1007/s11356-021-18044-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-18044-7