Abstract
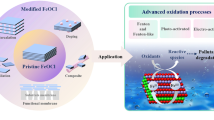
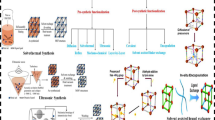
Heterogeneous Fenton-like catalysis mediated by solid catalyst is a promising oxidation technology for water purification. The redox reactivity, cost-effectiveness, and environmental compatibility of solid catalyst play governing roles in oxidant activation, radical generation, and pollutant degradation. Herein, the surface-disordered WO3 (D-WO3) functionally engineered by the unique crystalline-amorphous core–shell structure is proven to be a superior solid catalyst of heterogeneous Fenton-like catalysis for peroxymonosulfate (PMS) activation and pollutant degradation in various water matrices. Six typical phenolic and dye pollutants are effectively and selectively degraded in the D-WO3/PMS system with much reduced matrix effects. Both radical identifying and scavenging tests elucidate the important role of non-radical 1O2 and mediated electron transfer during PMS activation on the D-WO3 surface. The superior Fenton-like activity of D-WO3 can be mainly attributed to the surface and sub-surface distorted lattice sites with finely tailored atomic and electronic structures and surface chemistry. These distorted lattice sites can thermodynamically serve as the key reactive centers of dissociative adsorption and catalytic activation for both PMS and pollutant, with high adsorption energy, strong structural activation, and smooth electron transfer. Our findings provide a new chance for heterogeneous Fenton-like catalysis mediated by transition metal oxides with high capacity, low cost, and no toxicity for promising water purification.







Similar content being viewed by others
Data availability
The data sets used during the current study are available from the corresponding author on reasonable request.
References
Ahn Y-Y, Yun E-T, Seo J-W, Lee C, Kim SH, Kim J-H, Lee J (2016) Activation of peroxymonosulfate by surface-loaded noble metal nanoparticles for oxidative degradation of organic compounds. Environ Sci Technol 50:10187–10197
Anantharaj S, Noda S (2020) Amorphous catalysts and electrochemical water splitting: an untold story of harmony. Small 16:1905779
Anipsitakis GP, Dionysiou DD (2004) Radical generation by the interaction of transition metals with common oxidants. Environ Sci Technol 38:3705–3712
Antonopoulou M, Evgenidou E, Lambropoulou D, Konstantinou I (2014) A review on advanced oxidation processes for the removal of taste and odor compounds from aqueous media. Water Res 53:215–234
Awfa D, Ateia M, Fuji M, Johnson MS, Yoshimura C (2018) Photodegradation of pharmaceuticals and personal care products in water treatment using carbonaceous-TiO2 composites: a critical review of recent literature. Water Res 142:26–45
Behara DK, Ummireddi AK, Aragonda V, Gupta PK, Pala RGS, Sivakumar S (2016) Coupled optical absorption, charge carrier separation, and surface electrochemistry in surface disordered/hydrogenated TiO2 for enhanced PEC water splitting reaction. Phys Chem Chem Phys 188:364–8377
Duan XG, Sun HQ, Kang J, Wang YX, Indrawirawan S, Wang SB (2015a) Insights into heterogeneous catalysis of persulfate activation on dimensional-structured nanocarbons. ACS Catal 5:4629–4636
Duan XG, Sun HQ, Wang SB (2018) Metal-free carbocatalysis in advanced oxidation reactions. Acc Chem Res 51:678–687
Duan XG, Sun HQ, Wang YX, Kang J, Wang SB (2015b) N-doping-induced nonradical reaction on single-walled carbon nanotubes for catalytic phenol oxidation. ACS Catal 5:553–559
Guan CT, Jiang J, Pang SY, Luo CW, Ma J, Zhou Y, Yang Y (2017) Oxidation kinetics of bromophenols by nonradical activation of peroxydisulfate in the presence of carbon nanotube and formation of brominated polymeric products. Environ Sci Technol 51:10718–10728
Huang KZ, Zhang HC (2019) Direct electron-transfer-based peroxymonosulfate activation by iron-doped manganese oxide (δ-MnO2) and the development of galvanic oxidation processes. Environ Sci Technol 53:12610–12620
Huang Y, Kong MH, Coffin S, Cochran KH, Westerman DC, Schlenk D, Richardson SD, Lei LC, Dionysiou DD (2020) Degradation of contaminants of emerging concern by UV/H2O2 for water reuse: kinetics, mechanisms, and cytotoxicity analysis. Water Res 174:115587
Hu PD, Su HR, Chen ZY, Yu CY, Li QL, Zhou BX, Alvarez PJJ, Long MC (2017) Selective degradation of organic pollutants using an efficient metal-free catalyst derived from carbonized polypyrrole via peroxymonosulfate activation. Environ Sci Technol 51:11288–11296
Kim J, Lee CW, Choi W (2010) Platinized WO3 as an environmental photocatalyst that generates OH radicals under visible light. Environ Sci Technol 44:6849–6854
Koo MS, Chen XF, Cho K, An TC, Choi W (2019) In situ photoelectrochemical chloride activation using a WO3 electrode for oxidative treatment with simultaneous H2 evolution under visible light. Environ Sci Technol 53:9926–9936
Lee H, Kim H-i, Weon S, Choi W, Hwang YS, Seo J, Lee C, Kim J-H (2016) Activation of persulfates by graphitized nanodiamonds for removal of organic compounds. Environ Sci Technol 50:10134–10142
Lee H, Lee H-J, Jeong J, Lee J, Park N-B, Lee C (2015) Activation of persulfates by carbon nanotubes: oxidation of organic compounds by nonradical mechanism. Chem Eng J 266:28–33
Li XT, Ma JZ, Yang L, He GZ, Zhang CB, Zhang RD, He H (2018) Oxygen vacancies induced by transition metal doping in γ-MnO2 for highly efficient ozone decomposition. Environ Sci Technol 52:12685–12696
Lindsey ME, Tarr MA (2000) Inhibition of hydroxyl radical reaction with aromatics by dissolved natural organic matter. Environ Sci Technol 34:444–449
Liu C, Min Y, Zhang A-Y, Si Y, Chen J-J, Yu H-Q (2019) Electrochemical treatment of phenol-containing wastewater by facet-tailored TiO2: efficiency, characteristics and mechanisms. Water Res 165:114980
Liu Z, Ren BX, Ding HJ, He H, Deng HP, Zhao C, Wang P, Dionysiou DD (2020) Simultaneous regeneration of cathodic activated carbon fiber and mineralization of desorbed contaminations by electro-peroxydisulfate process: advantages and limitations. Water Res 171:115456
Luo XB, Deng F, Min LJ, Luo SL, Guo B, Zeng GS, Au C (2013) Facile one-step synthesis of inorganic-framework molecularly imprinted TiO2/WO3 nanocomposite and its molecular recognitive photocatalytic degradation of target contaminant. Environ Sci Technol 47:7404–7412
Miao J, Geng W, Alvarez PJJ, Long MC (2020) 2D N-doped porous carbon derived from polydopamine-coated graphitic carbon nitride for efficient nonradical activation of peroxymonosulfate. Environ Sci Technol 54:8473–8481
Ren W, Xiong LL, Yuan XH, Yu ZW, Zhang H, Duan XG, Wang SB (2019) Activation of peroxydisulfate on carbon nanotubes: electron-transfer mechanism. Environ Sci Technol 53:14595–14603
Sun ZQ, Zhao L, Liu CH, Zhen YF, Ma J (2019) Catalytic ozonation of ketoprofen with in situ N-doped carbon: a novel synergetic mechanism of hydroxyl radical oxidation and an intra-electron-transfer nonradical reaction. Environ Sci Technol 53:10342–10351
Westerhoff P, Aiken G, Amy G, Debroux J (1999) Relationships between the structure of natural organic matter and its reactivity towards molecular ozone and hydroxyl radicals. Water Res 33:2265–2276
Yang ZC, Qian JS, Yu AQ, Pan BC (2019) Singlet oxygen mediated iron-based Fenton-like catalysis under nanoconfinement. Proc Natl Acad Sci USA 116:6659–6664
Yao JY, Yu Y, Qu RJ, Chen J, Huo ZL, Zhu F, Wang ZY (2020) Fe-activated peroxymonosulfate enhances the degradation of dibutyl phthalate on ground quartz sand. Environ Sci Technol 54:9052–9061
Yun E-T, Lee JH, Kim J, Park H-D, Lee J (2018) Identifying the nonradical mechanism in the peroxymonosulfate activation process: singlet oxygenation versus mediated electron transfer. Environ Sci Technol 52:7032–7042
Yun E-T, Yoo H-Y, Bae H, Kim H-I, Lee J (2017) Exploring the role of persulfate in the activation process: radical precursor versus electron acceptor. Environ Sci Technol 51:10090–10099
Zhang A-Y, He Y-Y, Chen Y-P, Feng J-W, Huang N-H, Lian F (2020a) Degradation of organic pollutants by Co3O4-mediated peroxymonosulfate oxidation: roles of high-energy {001}-exposed TiO2 support. Chem Eng J 334:1430–1439
Zhang A-Y, Zhao P-C, He Y-Y, Zhou Y, Feng J-W (2020b) Non-radical activation of H2O2 by surface-disordered WO3 for efficient and selective pollutants degradation with weak matrix effects. Environ Sci Poll Res 27:1898–1911
Zhang T, Chen Y, Wang YR, Roux JL, Yang Y, Croué J-P (2014) Efficient peroxydisulfate activation process not relying on sulfate radical generation for water pollutant degradation. Environ Sci Technol 48:5868–5875
Zhu SS, Huang XC, Ma F, Wang L, Duan XG, Wang SB (2018) Catalytic removal of aqueous contaminants on N-doped graphitic biochars: inherent roles of adsorption and nonradical mechanisms. Environ Sci Technol 52:8649–8658
Funding
This work is supported by the National Natural Science Foundation of China (21876040, 22076036), the Fundamental Research Funds for the Central Universities (PA2021KCPY0031), and the Open Project of State Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology (No. ESK202105).
Author information
Authors and Affiliations
Contributions
AYZ, SX, and PCZ conceptualized and conducted the experiment. AYZ, HL, and JFW wrote the manuscript. AYZ and HL supervised the work. JWF and PCZ conducted the literature review and compiled the overall manuscript.
Corresponding authors
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ricardo A. Torres-Palma
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, AY., Xu, S., Feng, JW. et al. Superior degradation of phenolic contaminants in different water matrices via non-radical Fenton-like mechanism mediated by surface-disordered WO3. Environ Sci Pollut Res 29, 18259–18270 (2022). https://doi.org/10.1007/s11356-021-17088-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-17088-z