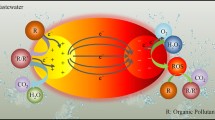
Abstract
Heterogeneous catalysis is promising for water treatment. Solid catalysts play governing roles. Herein, the surface-disordered WO3, D-WO3, engineered with surface and sub-surface defective sites from NaBH4 reduction was proven to be an effective catalyst for H2O2 activation. The defective degree and defects amount on WO3 were regulated by NaBH4. More than 95% of two typical azo dyes, RhB and MG, were selectively degraded in D-WO3/H2O2 system during 3.0 h, while no significant activity was observed for MO as well as bisphenol A, roxarsone, phenol, 4-chlorophenol, p-nitrophenol, o-aminophenol, urea, and 2,4-dichlorophenol in comparison under the identical conditions (mainly less than 20%). Both ESR and radical scavenging tests indicated the minor role of ·OH from H2O2 activation on D-WO3. The superior activity of D-WO3 could be mainly attributed to the surface and sub-surface defects with finely tailored local atomic configurations and electronic structures of central metal sites. Surface and sub-surface defective sites could serve as the reactive sites of interfacial adsorption, dissociative activation, and catalytic decomposition for both oxidant and pollutants, with high adsorption energy, strong structural activation, and superior catalytic activity. Our findings provided a new chance for non-selective radical catalysis based on transition metal oxides and a promising catalyst with high performance, low cost, and no toxicity for pollutant degradation with weak matrix effects in wastewater and surface water.











Similar content being viewed by others
References
Ahn Y-Y, Yun E-T, Seo J-W, Lee C, Kim SH, Kim J-H, Lee J (2016) Activation of peroxymonosulfate by surface-loaded noble metal nanoparticles for oxidative degradation of organic compounds. Environ Sci Technol 50:10187–10197
Anipsitakis GP, Dionysiou DD (2004) Radical generation by the interaction of transition metals with common oxidants. Environ Sci Technol 38:3705–3712
Antonopoulou M, Evgenidou E, Lambropoulou D, Konstantinou I (2014) A review on advanced oxidation processes for the removal of taste and odor compounds from aqueous media. Water Res 53:215–234
Behara DK, Ummireddi AK, Aragonda V, Gupta PK, Pala RGS, Sivakumar S (2016) Coupled optical absorption, charge carrier separation, and surface electrochemistry in surface disordered/hydrogenated TiO2 for enhanced PEC water splitting reaction. Phys Chem Chem Phys 18:8364–8377
Chen DJ, Chen C, Baiyee ZM, Shao ZP, Ciucci F (2015a) Nonstoichiometric oxides as low-cost and highly-efficient oxygen reduction/evolution catalysts for low-temperature electrochemical devices. Chem Rev 115:9869–9921
Chen XB, Liu L, Huang FQ (2015b) Black titanium dioxide (TiO2) nanomaterials. Chem Soc Rev 44:1861–1885
Cheng X, Guo HG, Zhang YL, Liu Y, Liu HW, Yang Y (2016) Oxidation of 2,4-dichlorophenol by non-radical mechanism using persulfate activated by Fe/S modified carbon nanotubes. J Colloid Interface Sci 469:277–286
Deguillaume L, Leriche M, Chaumerliac N (2005) Impact of radical versus non-radical pathway in the Fenton chemistry on the iron redox cycle in clouds. Chemosphere 60:718–724
Duan XG, Sun HQ, Kang J, Wang YX, Indrawirawan S, Wang SB (2015a) Insights into heterogeneous catalysis of persulfate activation on dimensional-structured nanocarbons. ACS Catal 5:4629–4636
Duan XG, Sun HQ, Wang YX, Kang J, Wang SB (2015b) N-doping-induced nonradical reaction on single-walled carbon nanotubes for catalytic phenol oxidation. ACS Catal 5:553–559
Duan XG, Ao ZM, Sun HQ, Zhou L, Wang GX, Wang SB (2015c) Insights into N-doping in single-walled carbon nanotubes for enhanced activation of superoxides: a mechanistic study. Chem Commun 51:15249–15252
Duan XG, Ao ZM, Zhou L, Sun HQ, Wang GX, Wang SB (2016) Occurrence of radical and nonradical pathways from carbocatalysts for aqueous and nonaqueous catalytic oxidation. Appl Catal B Environ 188:98–105
Gao YW, Zhu Y, Lyu L, Zeng QY, Xing XC, Hu C (2018) Electronic structure modulation of graphitic carbon nitride by oxygen doping for enhanced catalytic degradation of organic pollutants through peroxymonosulfate activation. Environ Sci Technol 52:14371–14380
Guan C-T, Jiang J, Luo C-W, Ma J, Pang S-Y, Jiang CC, Jin Y-X, Li J (2017) Transformation of iodide by carbon nanotube activated peroxydisulfate and formation of iodoorganic compounds in the presence of natural organic matter. Environ Sci Technol 51:479–487
Heckert EG, Seal S, Self WT (2008) Fenton-like reaction catalyzed by the rare earth inner transition metal cerium. Environ Sci Technol 42:5014–5019
Hu PD, Su HR, Chen ZY, Yu CY, Li QL, Zhou BX, Alvarez JJP, Long MC (2017) Selective degradation of organic pollutants using an efficient metal-free catalyst derived from carbonized polypyrrole via peroxymonosulfate activation. Environ Sci Technol 51:11288–11296
Khan JA, He XX, Khan HM, Shah NS, Dionysiou DD (2013) Oxidative degradation of atrazine in aqueous solution by UV/H2O2/Fe2+, UV/S2O82-/Fe2+ and UV/HSO5-/Fe2+ processes: a comparative study. Chem Eng J 218:376–383
Lee H, H-i K, Weon S, Choi W, Hwang YS, Seo J, Lee C, Kim J-H (2016) Activation of persulfates by graphitized nanodiamonds for removal of organic compounds. Environ Sci Technol 50:10134–10142
Lee H, Lee H-J, Jeong J, Lee J, Park N-B, Lee C (2015) Activation of persulfates by carbon nanotubes: oxidation of organic compounds by nonradical mechanism. Chem Eng J 266:28–33
Li H, Shang J, Yang ZP, Shen WJ, Ai ZH, Zhang LZ (2017) Oxygen vacancy associated surface Fenton chemistry: surface structure dependent hydroxyl radicals generation and substrate dependent reactivity. Environ Sci Technol 51:5685–5694
Li HC, Shan C, Li W, Pan BC (2018a) Peroxymonosulfate activation by iron(III)-tetraamidomacrocyclic ligand for degradation of organic pollutants via high-valent iron-oxo complex. Water Res 147:233–241
Li HC, Shan C, Pan BC (2018b) Fe(III)-doped g-C3N4 mediated peroxymonosulfate activation for selective degradation of phenolic compounds via high-valent ironoxo species. Environ Sci Technol 52:2197–2205
Li YH, Liu PF, Pan LF, Wang HF, Yang ZZ, Zheng LR, Hu P, Zhao HJ, Gu L, Yang HG (2015) Local atomic structure modulations activate metal oxide as electrocatalyst for hydrogen evolution in acidic water. Nat Commun 6:8064
Li XT, Ma JZ, Yang L, He GZ, Zhang CB, Zhang RD, He H (2018) Oxygen vacancies induced by transition metal doping in γ-MnO2 for highly efficient ozone decomposition. Environ Sci Technol 52:12685–12696
Liu L, Chen XB (2014) Titanium dioxide nanomaterials: self-structural modifications. Chem Rev 114:9890–9918
Nunes GS, Alexiou ADP, Toma HE (2008) Catalytic oxidation of hydrocarbons by trinuclear μ-oxo-bridged ruthenium-acetate clusters: radical versus non-radical mechanisms. J Catal 260:188–192
Qian XF, Ren M, Zhu Y, Yue DT, Han Y, Jia JP, Zhao YX (2017) Visible light assisted heterogeneous Fenton-like degradation of organic pollutant via α-FeOOH/mesoporous carbon composites: Environ. Sci Technol 51:3993–4000
Rastogi A, Al-Abed SR, Dionysiou DD (2009a) Sulfate radical-based ferrous-peroxymonosulfate oxidative system for PCBs degradation in aqueous and sediment systems., Appl. Catal. B 85:171–179
Rastogi A, Al-Abed SR, Dionysiou DD (2009b) Effect of inorganic, synthetic and naturally occurring chelating agents on Fe(II) mediated advanced oxidation of chlorophenols. Water Res 43:684–694
Saputra E, Muhammad S, Sun HQ, Ang HM, Tadé MO, Wang SB (2013) Different crystallographic one-dimensional MnO2 nanomaterials and their superior performance in catalytic phenol degradation. Environ Sci Technol 47:5882–5887
Shi F, Tse MK, Li ZP, Beller M (2008) Controlling iron-catalyzed oxidation reactions: from non-selective radical to selective non-radical reactions. Chem Eur J 14:8793–8797
Wang GM, Ling YC, Wang HY, Yang XY, Wang CC, Zhang JZ, Li Y (2012) Hydrogen-treated WO3 nanoflakes show enhanced photostability. Energy Environ Sci 5:6180–6187
Yun E-T, Lee JH, Kim J, Park H-D, Lee J (2018) Identifying the nonradical mechanism in the peroxymonosulfate activation process: singlet oxygenation versus mediated electron transfer. Environ Sci Technol 52:7032–7042
Yun E-T, Yoo H-Y, Bae H, Kim H-I, Lee J (2017) Exploring the role of persulfate in the activation process: radical precursor versus electron acceptor. Environ Sci Technol 51:10090–10099
Zhang A-Y, Huang N-H, Zhang C, Zhao P-C, Lin T, He Y-Y, Feng J-W (2018) Heterogeneous Fenton decontamination of organoarsenicals and simultaneous adsorption of released arsenic with reduced secondary pollution. Chem Eng J 344:1–11
Zhang T, Chen Y, Wang YR, Roux JL, Yang Y, Croué J-P (2014) Efficient peroxydisulfate activation process not relying on sulfate radical generation for water pollutant degradation. Environ Sci Technol 48:5868–5875
Zhang T, Li WW, Croué J-P (2011) Catalytic ozonation of oxalate with a cerium supported palladium oxide: an efficient degradation not relying on hydroxyl radical oxidation. Environ Sci Technol 45:9339–9346
Zhu NW, Gu L, Yuan HP, Lou ZY, Wang L, Zhang X (2012) Degradation pathway of the naphthalene azo dye intermediate 1-diazo-2-naphthol-4-sulfonic acid using Fenton’s reagent. Water Res 46:3859–3867
Zhu SS, Huang XC, Ma F, Wang L, Duan XG, Wang SB (2018) Catalytic removal of aqueous contaminants on N-doped graphitic biochars: inherent roles of adsorption and nonradical mechanisms. Environ Sci Technol 52:8649–8658
Zhou Y, Jiang J, Gao Y, Ma J, Pang S-Y, Li J, Lu X-T, Yuan L-P (2015) Activation of peroxymonosulfate by benzoquinone: a novel nonradical oxidation process. Environ Sci Technol 49:12941–12950
Funding
This work is supported by the National Natural Science Foundation of China (21876040), the Fundamental Research Funds for the Central Universities (PA2019GDQT0010), the Anhui Provincial Natural Science Foundation (1708085MB52), and the special funds from State Key Laboratory of Pollution Control and Resource Reuse (PCRRF17003) and State Key Joint Laboratory of Environment Simulation and Pollution Control (18K09ESPCT).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Vítor Pais Vilar
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 5569 kb)
Rights and permissions
About this article
Cite this article
Zhang, AY., Zhao, PC., He, YY. et al. Non-radical activation of H2O2 by surface-disordered WO3 for efficient and selective pollutant degradation with weak matrix effects. Environ Sci Pollut Res 27, 1898–1911 (2020). https://doi.org/10.1007/s11356-019-06899-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06899-w