Abstract
Few studies have evaluated the short-term association between hospital admissions and individual exposure to ambient particulate matter (PM2.5). Particularly, no studies focused on hospital admissions for chronic obstructive pulmonary disease (COPD) at the individual level. We assessed the short-term effects of PM2.5 on hospitalization admissions for COPD in Guangzhou, China, during 2014–2015, based on satellite-derived estimates of ambient PM2.5 concentrations at a 1-km resolution near the residential address as individual-level exposure for each patient. Around 40,002 patients with COPD admitted to 110 hospitals were included in this study. A time-stratified case-crossover design with conditional logistic regression models was applied to assess the effects of PM2.5 based on a 1-km grid data of aerosol optical depth provided by the National Aeronautics and Space Administration on hospital admissions for COPD. Further, we performed stratified analyses by individual demographic characteristics and season of hospital admission. Around 10 μg/m3 increase in individual-level PM2.5 was associated with an increase of 1.6% (95% confidence interval [CI]: 0.6%, 2.7%) in hospitalization for COPD at a lag of 0–5 days. The impact of PM2.5 on hospitalization for COPD was greater significantly in males and patients admitted in summer. Our study strengthened the evidence for the adverse effect of PM2.5 based on satellite-based individual-level exposure data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fine particulate matter with aerodynamic diameters < 2.5 μm (PM2.5) is a great concern for public health since it can penetrate into the lung deeply and induce respiratory diseases (Chen et al. 2017; Jiang et al. 2016; Kloog et al. 2012; Liu et al. 2018; Yang et al. 2020). Chronic obstructive pulmonary disease (COPD) is a major chronic respiratory disease, and the prevalence of COPD reached 251 million globally in 2016 (Hopke et al. 2019; Salimi et al. 2018; World Health Organization 2017). Elucidating the impact of exposure to PM2.5 on hospitalizations for COPD can help for developing interventions and thereafter reducing the burden of diseases.
An increasing number of studies have associated exposure to PM2.5 with hospital admissions for respiratory diseases, including COPD (Harrison and Yin 2000; Hopke et al. 2019; Kim et al. 2015; Lin et al. 2020). However, the estimates of the association between PM2.5 and hospital admissions were not always consistent (Belleudi et al. 2010; Kim et al. 2015; Kloog et al. 2014; Ko et al. 2007; Lin et al. 2020; Rice et al. 2015). Geographical variations in the estimates of associations suggested in prior studies may be due to the disparities in the level of air pollutant concentration, regional climates and socio-economical levels, and characteristics of local residents (Kumar et al. 2018). Thus, reports of different study locations can be beneficial for delineating a comprehensive contour of the adverse health effect of PM2.5.
More importantly, previous studies examined health effects of ambient PM2.5 exposure by using the PM2.5 level of the nearest station as individual exposure or more commonly using the average PM2.5 level of a few monitoring stations as a proxy of exposure for the whole population (Harrison and Yin 2000; Hopke et al. 2019; Kim et al. 2015; Lin et al. 2020; Santus et al. 2012; Zhang et al. 2019). The limited number of monitoring stations is not necessarily representative enough for the exposure level of the population in a location and thus there may be bias in such proxy (Goldman et al. 2011; Liu et al. 2017; Samet et al. 2000). In addition, the PM2.5 level calculated from selected monitoring stations ignores the spatial gradient of the exposure level among patients which may lead to misclassification of the exposure as well (Faustini et al. 2012; Liu et al. 2018; Moolgavkar and Suresh 2000; Tian et al. 2018). With the development of satellite monitoring technology, some models have been developed to estimate the level of ambient PM2.5 based on satellite data (Danesh et al. 2019; Guo et al. 2018; Lin et al. 2018). We previously proposed a method for a high-coverage and accurate estimation of daily PM2.5 at a 1-km resolution in mainland China based on the satellite data of atmospheric aerosol optical depth (AOD) by solving the problem of high missing rate, which is usually ignored in previous studies and leads to inaccurate estimation of PM2.5(Chen et al. 2020). The 1-km estimates of PM2.5 which can be matched with the residential addresses are expected to be a more precise proxy for the exposure level of PM2.5 for the subjects involved in studies on the health effect of air pollution, and the effect estimates tend to be more reliable (Hennig et al. 2020; Wong et al. 2015; Woo et al. 2020). Several cohort studies have applied satellite-derived exposure data to quantify the long-term effects of PM2.5 on health outcomes, with the data of addresses of subjects (Danesh et al. 2019; Guo et al. 2018; Lin et al. 2018). To our knowledge, there is no previous study assessing the short-term effects of PM2.5 on hospital admissions for COPD using satellite-based estimates of PM2.5 at a 1-km resolution or higher as individual exposure level, although Kloog et al. (2014) estimated the short-term effects on all-cause hospital admissions using PM2.5 at a 10 × 10-km spatial resolution in the Mid-Atlantic.
Guangzhou is one of the largest cities in the south of China (latitude: 23°07′N; longitude 113°15′E), covering an area of 7434.4 km2 with 14.9 million permanent residents. The average annual PM2.5 concentration in Guangzhou was from 37.0 to 46.8 μg/m3 between 2014 and 2015, which was much higher than the air quality guideline of WHO, 10 μg/m3 (World Health Organization 2005). The present study aims to assess the short-term effects of ambient PM2.5 on hospital admissions for COPD in Guangzhou, China, during 2014–2015, based on satellite-derived estimates of daily PM2.5 concentrations at a 1-km resolution near the residential address as individual-level exposure for each individual.
Materials and methods
Data sources
The data on the home page of electronic medical records for all individual hospitalization due to COPD admitted in 110 hospitals during 2014–2015 in Guangzhou were retrieved from the Guangzhou Health Information Center, which is a part of the National Health Statistics Network Direct Report system having standard operation procedures and sophisticated measures for quality control (National Health and Family Planning Commission of the People's Republic of China 2007). The database of this system of Guangzhou encompassed all 122 hospitals ranked level II and above, in which 110 hospitals admitting COPD patients were used in this research. Principal diagnosis was coded according to the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10). We extracted the information for all individuals admitted due to COPD (ICD-10 code: J40-J44), including sex, age, residential address, occupational class, marital status, and date of admission. Daily meteorological data, including daily mean temperature and relative humidity, were downloaded from the National Meteorological Center (http://data.cma.cn/).
The study was approved by the ethical committee of Southern Medical University, and patient informed consent was waived since only de-identified data derived from the official health information system were provided and analyzed anonymously.
Exposure assessment
In our previous study, we applied extreme gradient boosted (XGBoost) imputation to fill the missing gap of a 1-km multiangle implementation of AOD obtained from the National Aeronautics and Space Administration, and the coverage of AOD was significantly increased from 15.46 to 98.64% on average in mainland China (Chen et al. 2020). Then a combined method of non-linearexposure-lag-response model and XGBoost was applied to predict daily 1 × 1-km PM2.5 concentrations using meteorological variables and the XGBoost-interpolated AOD, with high predictive accuracy (cross-validation R2 = 0.81 for Guangzhou) (Chen et al. 2020). Daily levels of PM2.5 were well estimated for all grids including those without monitoring stations. Next, each patient’s residential address was matched to the 1-km grid cell according to the longitude and latitude and then the corresponding daily PM2.5 concentrations on the current day of admission and the previous 6 days before admission in the grid cell were treated as the proxy of daily exposure for the patient.
Statistical analysis
We adopted a time-stratified case-crossover design to assess the associations between PM2.5 and hospital admissions for COPD. This approach has been widely applied to evaluate the health effects of air pollution in which each case serves as his/her own control (Levy et al. 2001; Szyszkowicz et al. 2018; Tsai et al. 2013). In this study, the case days were defined as the dates of admission, while the referent days were those that fell on the same day of the week within the same month as the case day. Since the case period and the referent period are very close in time, this design avoids the influence of individual characteristics and long-term and seasonal trends on the effect estimation (Bateson and Schwartz 1999; Janes et al. 2005).
We used conditional logistic regression models to quantify the impacts of PM2.5 on hospitalizations due to COPD. The model was of the following form:
Pi and αi are the probability of hospitalization and the intercept for stratum i. According to previous studies, a natural cubic spline (NS) with three degrees of freedom (dfs) was applied for the moving average of temperature (T) and relative humidity (RH) at a lag of 0–6 days (Liu et al. 2017; Lu et al. 2019; Tian et al. 2018). In addition, an indicator variable of public holidays (Holiday) was also included (Hwang et al. 2017; Ma et al. 2019). δ is the regression coefficient for Holiday. Here, we considered the effects of PM2.5 on hospitalizations for COPD at lags up to 6 days. Both single-day and multiple-day lags were considered in this study (Devries et al. 2016; Hwang et al. 2017; Liu et al. 2018).
Next, we conducted stratified analysis by sex, age (<65 and ≥65 years), occupational class (the unemployed, blue-collar workers, and white-collar workers), marital status (married, unmarried, and divorced/widowed), and season of hospital admission (winter [December to February], spring [March to May], summer [June to August], and autumn [September to November]) (Ma et al. 2019). The differences in the effects of PM2.5 on hospital admissions for COPD between two subgroups were tested via the Z test with the following formula:
where β1 and β2 represent the regression coefficients of PM2.5 for two subgroups and SE(β1) and SE(β2) are the corresponding standard errors. Sensitivity analyses were conducted to assess the robustness of the results by changing dfs for temperature and relative humidity from 3 to 2–6 and using different lag days for meteorology with lag 0–14 and lag 0–21.
All statistical tests were two-sided, and P < 0.05 was considered statistically significant. We performed all data analyses using R software (version 3.5.1, R Foundation for Statistical Computing).
Results
A total of 40,002 patients admitted to 110 hospitals with COPD from 2014 to 2015 were included in the analysis (Fig. 1). Patients hospitalized for COPD were predominately males (74.1%) and those aged ≥65 years (83.8%) (Table 1). The unemployed and married individuals accounted for 81.5% and 93.3% of the hospital admissions for COPD. It seemed that the differences in the proportions of hospitalizations for COPD across four seasons were small (Table 1). Average satellite-derived1-km-resolution PM2.5 concentrations during the study period were 42.6 μg/m3 (ranges from 6.0 to 164.4 μg/m3) for COPD patients. Daily temperature and relative humidity were on average 22.2°C (ranges from 5.2 to 31.4°C) and 79.8% (ranges from 31.5 to 97.5%) during the study period (Table 2).
The effect of PM2.5 on hospital admission for COPD was higher on the current day in the single-day model, meanwhile the effect was the greatest for the moving average of 0–5 days than for other lagged days (Table 3). It was estimated that a 10-μg/m3 increase in PM2.5 was associated with an increase of 1.6% (OR = 1.016, 95% confidence interval [CI]: 1.006, 1.027) in hospitalization for COPD at a lag of 0–5 days.
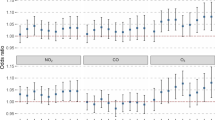
We estimated the ORs of hospitalizations for COPD per 10-μg/m3 increase in individual exposure level of PM2.5, at lag 0–5 days, for different subgroups. The effect of PM2.5 on hospital admission for COPD was statistically significant for males (OR: 1.023, 95% CI: 1.011, 1.036), while the effect was statistically non-significant for females, and the difference in the effects of PM2.5 was statistically significant by sex (P = 0.046). It was witnessed that hospitalizations for COPD (OR: 1.020, 95% CI: 1.008, 1.032) increased with PM2.5 among people ≥65 years of age. However, the difference in the effects of PM2.5 was not statistically significant by age (P > 0.05). The effect of PM2.5 on hospitalizations for COPD seemed not to vary by occupational class and marital status (P > 0.05). A larger OR (1.052 [95% CI: 1.021, 1.084]) was estimated for patients admitted due to COPD in summer than in other seasons (P = 0.048) (Fig. 2 and Table S1).
Odds ratios of hospitalizations for chronic obstructive pulmonary disease (COPD) per 10-μg/m3 increase of individual exposure level of PM2.5 at a lag of 0–5 days in subgroup analysis. Points and lines are point estimates and the corresponding 95% confidence interval of odds ratios. The dashed line indicates odds ratio = 1
According to the results of sensitivity analysis, the effect estimates were not substantially changed when changing the dfs or lag days for temperature and relative humidity from 3 to 2–6(Table S2 and Table S3).
Discussion
We estimated the short-term effect of ambient PM2.5 on hospital admissions for COPD based on satellite-derived1-km-resolution estimates of PM2.5 in Guangzhou, China. We found a significant association between hospital admissions for COPD with the ambient PM2.5 at individual exposure level, and the effect of PM2.5 was stronger in males and patients admitted during the warm season. Our findings provided more solid evidence for the association between ambient PM2.5 and hospital admissions for COPD with individual exposure data.
In this study, we estimated that a 10-μg/m3 increase in PM2.5 was associated with an increase of 1.6% in hospitalization for COPD at a lag of 0–5 days. Statistically significant associations between PM2.5 and hospital admissions for COPD were also reported in other studies using ground-level monitoring data of PM2.5, but the magnitude of estimates varied across study locations. For example, the corresponding effect estimates for COPD admissions were 2.06% at lag 0–6 days in Ningbo, China (Zhang et al. 2019) and 3.10% at lag 0–2 days in Milan, Italy (Santus et al. 2012). Lin et al. (2020) showed that the effect of PM2.5 on hospital admissions for COPD was highest at a distributed lag of 0–7 days (relative risks = 1.073, 95% CI: 1.016, 1.133) in Yinzhou District, China. Inconsistency was further observed in studies which reported statistically non-significant associations of PM2.5 with hospital admissions for COPD in some other cities (Belleudi et al. 2010; Liu et al. 2018; Peel et al. 2005; Slaughter et al. 2005; Stieb et al. 2009). A certain number of previous studies examined the associations with the data of a single or a few hospitals in which the sample size may be insufficient for the inference, and therefore, non-significant results were obtained (Peel et al. 2005; Slaughter et al. 2005). Previous studies on short-term associations between exposure to air pollutants and health outcomes commonly used the average measurements obtained from a few monitoring stations as the proxy of exposure (Lin et al. 2020; Santus et al. 2012; Tian et al. 2018; Zuo et al. 2019). Measurements of air pollutant concentrations obtained from monitoring stations can capture the temporal variation of the average exposure level. However, using the average measurements from a limited number of monitoring stations cannot fully reflect the spatial variation in exposure level of subjects which may lead to bias in the estimates of associations between air pollutants and health outcomes due to the measurement error of exposure (Hwang et al. 2017; Liu et al. 2017; Tian et al. 2018; Xie et al. 2019; Zuo et al. 2019). On this issue, an accurate proxy of individual exposure using satellite-based PM2.5 with high resolution is crucial when investigating the health effects of air pollution. In addition, different levels of air pollutant concentrations, demographic characteristics and healthcare facilities, and the criteria for hospital admission may explain the variations in estimates of the associations between PM2.5 and hospital admissions for COPD (Kumar et al. 2018). The plausible mechanism that PM2.5 could affect COPD was the airway irritation leading to interactions with the immune system to cause respiratory infections, oxidative stress, and pulmonary inflammation (Mu et al. 2014; Szyszkowicz et al. 2018). In addition, the toxicity and irritability of particulate matter may aggravate lung infection, reducing the immune efficacy of the lung (Tsai et al. 2013).
Exploring the potential effect modifiers of PM2.5 on hospital admissions can be helpful for identifying potentially susceptible populations and for developing a more accurately targeted intervention. The differences in the effects of PM2.5 on hospital admissions for COPD by sex were statistically significant in our study, which was in accordance with prior studies (Lin et al. 2018). A higher effect was observed in males, and the difference between gender may be due to lifestyle behaviors (tobacco and alcohol consumption, exercise, and diet) (Xie et al. 2019). As for the disparities by age, we found that the effects of PM2.5 on hospitalizations were statistically significant in individuals ≥65 years of age for COPD, while non-significant results were observed in those <65 years. Such finding was also observed in a study which explored the association between PM2.5 and hospital admissions for acute exacerbation of COPD in southwestern Taiwan, China (Hwang et al. 2017). A study in Hefei, China, claimed that the effect of PM2.5 on COPD hospitalization differed by age, although the statistical significance of the difference was not examined (Xie et al. 2019). Regarding the disparities in the associations of PM2.5 with hospitalizations for COPD by occupational class and marital status, a relatively small sample size for some subgroups in the present study may account for the lack of statistical power to detect significant effects.
We found that the effects of PM2.5 on hospitalizations for COPD varied by season, with the highest estimates of the effects occurring in summer. The variation in the effects of PM2.5 by season could be due to that people tend to participate in outdoor activities and may open windows more frequently during warm months, leading to more exposure to ambient air pollutants (Stafoggia et al. 2016; Tian et al. 2018). In the context of global warming, the temperature is expected to increase, and the heat wave will occur more frequently (Lee et al. 2018; Yang et al. 2021; Zhang et al. 2017). Consequently, adverse health impacts of exposure to PM2.5 should be paid more attention to in the future given the higher effects of PM2.5 on hospitalizations for COPD observed in summer. Further studies are required to clarify the underlying mechanism of the variation in effects of PM2.5 by season and then inform better preparedness for the potentially elevated burden associated with PM2.5 in the future.
The current study was subject to several limitations. First, we did not assess the effects of exposure to other air pollutants on hospitalizations for COPD since the individual exposure data tailored for each patient were not available. Second, the sample sizes were relatively small for some subgroups which may be insufficient for the inference of the effects of PM2.5 for these subgroups. Third, the current study controlled for the effects of temperature, relative humidity, and holiday on hospitalizations for COPD, but other potentially influential factors, such as respiratory virus activity, were not considered because of the unavailability of data. Fourth, although compared with the PM2.5 averaged from the monitoring stations, the satellite-based PM2.5 is more accurately reflected on the spatial gradient at a 1 × 1-km solution, this address-level exposure estimation is still a proxy of individual exposure to outdoor PM2.5, and we cannot rule out the residual measurement error. Further studies can be conducted to achieve more accurate estimates of the effects of PM2.5 with the use of personal exposure data accounting for both outdoor and indoor exposure levels.
Conclusions
We found a significant association between hospital admissions for COPD with the ambient PM2.5, and our study strengthened the evidence for the adverse effect of PM2.5 based on satellite-based individual-level exposure data. The impact of PM2.5 on hospitalization for COPD was greater in males and patients admitted in summer.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bateson TF, Schwartz J (1999) Control for seasonal variation and time trend in case-crossover studies of acute effects of environmental exposures. Epidemiology (Cambridge, Mass) 10:539–544
Belleudi V, Faustini A, Stafoggia M, Cattani G, Marconi A, Perucci CA, Forastiere F (2010) Impact of fine and ultrafine particles on emergency hospital admissions for cardiac and respiratory diseases. Epidemiology 21:414–423
Chen R, Yin P, Meng X, Liu C, Wang L, Xu X, Ross JA, Tse LA, Zhao Z, Kan H, Zhou M (2017) Fine particulate air pollution and daily mortality. A nationwide analysis in 272 Chinese cities. Am J Respir Crit Care Med 196:73–81
Chen Z, Jin J, Zhang R, Zhang T, Chen J, Yang J, Ou C, Guo Y (2020) Comparison of different missing-imputation methods for MAIAC (multiangle implementation of atmospheric correction) AOD in estimating daily PM2.5 levels. Remote Sens 12:3008
Danesh YM, Wang Y, Di Q, Zanobetti A, Schwartz J (2019)Long-term exposure to PM2.5 and ozone and hospital admissions of Medicare participants in the Southeast USA. Environ Int 130:104879
DeVries R, Kriebel D, Sama S (2016) Low level air pollution and exacerbation of existing COPD: a case crossover analysis. Environ Health 15:98
Faustini A, Stafoggia M, Cappai G, Forastiere F (2012)Short-term effects of air pollution in a cohort of patients with chronic obstructive pulmonary disease. Epidemiology 23:861–879
Goldman GT, Mulholland JA, Russell AG, Strickland MJ, Klein M, Waller LA, Tolbert PE (2011) Impact of exposure measurement error in air pollution epidemiology: effect of error type in time-series studies. Environ Health 10:61
Guo C, Zhang Z, Lau A, Lin CQ, Chuang YC, Chan J, Jiang WK, Tam T, Yeoh EK, Chan TC, Chang LY, Lao XQ (2018) Effect of long-term exposure to fine particulate matter on lung function decline and risk of chronic obstructive pulmonary disease in Taiwan: a longitudinal, cohort study. Lancet Planet Health 2:e114–e125
Harrison RM, Yin J (2000) Particulate matter in the atmosphere: which particle properties are important for its effects on health? Sci Total Environ 249:85–101
Hennig F, Geisel MH, Kälsch H, Lucht S, Mahabadi AA, Moebus S, Erbel R, Lehmann N, Jöckel K, Scherag A, Hoffmann B (2020) Air pollution and progression of atherosclerosis in different vessel beds—results from a prospective cohort study in the Ruhr area, Germany. Environ Health Perspect 128:107003
Hopke PK, Croft D, Zhang W, Lin S, Masiol M, Squizzato S, Thurston SW, van Wijngaarden E, Utell MJ, Rich DQ (2019) Changes in the acute response of respiratory diseases to PM2.5 in New York State from 2005 to 2016. Sci Total Environ 677:328–339
Hwang S, Lin Y, Guo S, Chou C, Lin C, Chi M (2017) Fine particulate matter on hospital admissions for acute exacerbation of chronic obstructive pulmonary disease in southwestern Taiwan during 2006–2012. Int J Environ Health Res 27:95–105
Janes H, Sheppard L, Lumley T (2005)Case-crossover analyses of air pollution exposure data. Epidemiology 16:717–726
Jiang X, Mei X, Feng D (2016) Air pollution and chronic airway diseases: what should people know and do? J Thorac Dis 8:E31–E40
Kim K, Kabir E, Kabir S (2015) A review on the human health impact of airborne particulate matter. Environ Int 74:136–143
Kloog I, Coull BA, Zanobetti A, Koutrakis P, Schwartz JD (2012) Acute and chronic effects of particles on hospital admissions in New-England. PLoS One 7:e34664
Kloog I, Nordio F, Zanobetti A, Coull BA, Koutrakis P, Schwartz JD (2014) Short term effects of particle exposure on hospital admissions in the mid-atlantic states: a population estimate. PLoS One 9:e88578
Ko FWS, Tam W, Wong TW, Chan DPS, Tung AH, Lai CKW, Hui DSC (2007) Temporal relationship between air pollutants and hospital admissions for chronic obstructive pulmonary disease in Hong Kong. Thorax 62:780–785
Kumar LD, Han LJ, Cheol KH (2018) Associations between ambient particulate matter and nitrogen dioxide and chronic obstructive pulmonary diseases in adults and effect modification by demographic and lifestyle factors. Int J Environ Res Public Health 15:363
Lee W, Choi HM, Lee JY, Kim DH, Honda Y, Kim H (2018) Temporal changes in mortality impacts of heat wave and cold spell in Korea and Japan. Environ Int 116:136–146
Levy D, Lumley T, Sheppard L, Kaufman J, Checkoway H (2001) Referent selection in case-crossover analyses of acute health effects of air pollution. Epidemiology (Cambridge, Mass) 12:186–192
Lin H, Qian ZM, Guo Y, Zheng Y, Ai S, Hang J, Wang X, Zhang L, Liu T, Guan W, Li X, Xiao J, Zeng W, Xian H, Howard SW, Ma W, Wu F (2018) The attributable risk of chronic obstructive pulmonary disease due to ambient fine particulate pollution among older adults. Environ Int 113:143–148
Lin C, Li D, Lu J, Yu Z, Zhu Y, Shen P, Tang M, Jin M, Lin H, Shui L, Chen K, Wang J (2020)Short-term associations between ambient fine particulate matter pollution and hospital visits for chronic obstructive pulmonary disease in Yinzhou District, China. Environ Sci Pollut Res 27(17):21647–21653
Liu Y, Xie S, Yu Q, Huo X, Ming X, Wang J, Zhou Y, Peng Z, Zhang H, Cui X, Xiang H, Huang X, Zhou T, Chen W, Shi T (2017)Short-term effects of ambient air pollution on pediatric outpatient visits for respiratory diseases in Yichang city, China. Environ Pollut 227:116–124
Liu Y, Sun J, Gou Y, Sun X, Li X, Yuan Z, Kong L, Xue F (2018) A multicity analysis of the short-term effects of air pollution on the chronic obstructive pulmonary disease hospital admissions in Shandong, China. Int J Environ Res Public Health 15:774
Lu P, Zhang Y, Lin J, Xia G, Zhang W, Knibbs LD, Morgan GG, Jalaludin B, Marks G, Abramson M, Li S, Guo Y (2019)Multi-city study on air pollution and hospital outpatient visits for asthma in China. Environ Pollut 257:113638
Ma Y, Yu Z, Jiao H, Zhang Y, Ma B, Wang F, Zhou J (2019)Short-term effect of PM2.5 on pediatric asthma incidence in Shanghai, China. Environ Sci Pollut Res 26:27832–27841
Moolgavkar, Suresh H (2000) Air pollution and hospital admissions for chronic obstructive pulmonary disease in three metropolitan areas in the United States. Inhal Toxicol 12:75–90
Mu L, Deng F, Tian L, Li Y, Swanson M, Ying J, Browne RW, Rittenhouse-Olson K, Zhang JJ, Zhang Z, Bonner MR (2014) Peak expiratory flow, breath rate and blood pressure in adults with changes in particulate matter air pollution during the Beijing Olympics: A panel study. Environ Res 133:4–11
National Health and Family Planning Commission of the People's Republic of China (2007) Notice of the General Office of the Ministry of Health on implementing the direct reporting work of the National Health Statistics Network.
World Health Organization (2005) WHO air quality guidelines for particulate matter, ozone, nitrogen dioxide and sulfur dioxide. Global Update 2005. Summary of Risk Assessment.
World Health Organization, 2017. COPD. https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd). Accessed 14 Aug 2020
Peel JL, Tolbert PE, Klein M, Metzger KB, Flanders WD, Todd K, Mulholland JA, Ryan PB, Frumkin H (2005) Ambient air pollution and respiratory emergency department visits. Epidemiology 16:164–174
Rice MB, Ljungman PL, Wilker EH, Dorans KS, Gold DR, Schwartz J, Koutrakis P, Washko GR, O'Connor GT, Mittleman MA (2015)Long-term exposure to traffic emissions and fine particulate matter and lung function decline in the Framingham heart study. Am J Respir Crit Care Med 191:656–664
Salimi F, Morgan G, Rolfe M, Samoli E, Cowie CT, Hanigan I, Knibbs L, Cope M, Johnston FH, Guo Y, Marks GB, Heyworth J, Jalaludin B (2018)Long-term exposure to low concentrations of air pollutants and hospitalisation for respiratory diseases: a prospective cohort study in Australia. Environ Int 121:415–420
Samet JM, Dominici F, Zeger SL, Schwartz J, Dockery DW (2000) The national morbidity, mortality, and air pollution study. Res Rep Health Eff Inst 94(5-14):75–84
Santus P, Russo A, Madonini E, Allegra L, Blasi F, Centanni S, Miadonna A, Schiraldi G, Amaducci S (2012) How air pollution influences clinical management of respiratory diseases. A case-crossover study in Milan. Respir Res 13:95
Slaughter JC, Kim E, Sheppard L, Sullivan JH, Larson TV, Claiborn C (2005) Association between particulate matter and emergency room visits, hospital admissions and mortality in Spokane, Washington. J Expo Anal Environ Epidemiol 15:153–159
Stafoggia M, Zauli-Sajani S, Pey J, Samoli E, Alessandrini E, Basagaña X, Cernigliaro A, Chiusolo M, Demaria M, Díaz J, Faustini A, Katsouyanni K, Kelessis AG, Linares C, Marchesi S, Medina S, Pandolfi P, Pérez N, Querol X et al (2016) Desert dust outbreaks in Southern Europe: contribution to daily PM10 concentrations and short-term associations with mortality and hospital admissions. Environ Health Perspect 124:413–419
Stieb DM, Szyszkowicz M, Rowe BH, Leech JA (2009) Air pollution and emergency department visits for cardiac and respiratory conditions: a multi-city time-series analysis. Environ Health 8:25
Szyszkowicz M, Kousha T, Castner J, Dales R (2018) Air pollution and emergency department visits for respiratory diseases: a multi-city case crossover study. Environ Res 163:263–269
Tian Y, Xiang X, Juan J, Song J, Cao Y, Huang C, Li M, Hu Y (2018)Short-term effects of ambient fine particulate matter pollution on hospital visits for chronic obstructive pulmonary disease in Beijing, China. Environ Health 17
Tsai S, Chang C, Yang C (2013) Fine particulate air pollution and hospital admissions for chronic obstructive pulmonary disease: a case-crossover study in Taipei. Int J Environ Res Public Health 10:6015–6026
Wong CM, Lai HK, Tsang H, Thach TQ, Thomas GN, Lam KBH, Chan KP, Yang L, Lau AKH, Ayres JG, Lee SY, Man Chan W, Hedley AJ, Lam TH (2015)Satellite-based estimates of long-term exposure to fine particles and association with mortality in elderly Hong Kong residents. Environ Health Perspect 123:1167–1172
Woo KS, Chook P, Hu YJ, Lao XQ, Lin CQ, Lee PWA, Kwok CYT, Wei AN, Guo DS, Yin YH, Lau KHA, Leung KS, Leung Y, Celermajer DS (2020) The impact of particulate matter air pollution (PM2.5) on atherosclerosis in modernizing China: a report from the CATHAY study. Int J Epidemiol
Xie J, Teng J, Fan Y, Xie R, Shen A (2019) The short-term effects of air pollutants on hospitalizations for respiratory disease in Hefei, China. Int J Biometeorol 63:315–326
Yang J, Zhou M, Li M, Yin P, Hu J, Zhang C, Wang H, Liu Q, Wang B (2020) Fine particulate matter constituents and cause-specific mortality in China: a nationwide modelling study. Environ Int 143:105927
Yang J, Zhou M, Ren Z, Li M, Wang B, Liu DL, Ou C, Yin P, Sun J, Tong S, Wang H, Zhang C, Wang J, Guo Y, Liu Q (2021) Projecting heat-related excess mortality under climate change scenarios in China. Nature Communications 12:1–11
Zhang R, Chen Z, Ou C, Zhuang Y (2017) Trends of heat waves and cold spells over 1951–2015 in Guangzhou, China. Atmosphere 8:37
Zhang Z, Chai P, Wang J, Ye Z, Shen P, Lu H, Jin M, Gu M, Li D, Lin H, Chen K (2019) Association of particulate matter air pollution and hospital visits for respiratory diseases: a time-series study from China. Environ Sci Pollut Res 26:12280–12287
Zuo B, Liu C, Chen R, Kan H, Sun J, Zhao J, Wang C, Sun Q, Bai H (2019) Associations between short-term exposure to fine particulate matter and acute exacerbation of asthma in Yancheng, China. Chemosphere 237:124497
Funding
This work was supported by the National Natural Science Foundation of China (81573249, 82003555), and the China Postdoctoral Science Foundation (2020M672744, 2020TQ0135).
Author information
Authors and Affiliations
Contributions
Jie-Qi Jin: conceptualization, writing—original draft, and formal analysis. Dong Han: resources and writing—review and editing. Qi Tian: writing—review and editing. Zhao-Yue Chen: resources and writing—review and editing. Yun-Shao Ye: resources and writing—review and editing. Qiao-Xuan Lin: writing—review and editing. Chun-Quan Ou: conceptualization, writing—review and editing, and supervision. Li Li: writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the ethical committee of Southern Medical University, and patient informed consent was waived since only de-identified data were collected and the data were analyzed anonymously.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Capsule
Short-term effects of PM2.5 on hospitalization admissions for COPD in individual level
Supplementary Information
ESM 1
(DOCX 39 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jin, JQ., Han, D., Tian, Q. et al. Individual exposure to ambient PM2.5 and hospital admissions for COPD in 110 hospitals: a case-crossover study in Guangzhou, China. Environ Sci Pollut Res 29, 11699–11706 (2022). https://doi.org/10.1007/s11356-021-16539-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-16539-x