Abstract
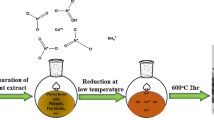
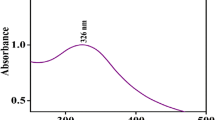
In this study, the efficiency of cerium oxide nanoparticles (CONPs) was examined for the adsorptive removal of various spectral indices of Natural Organic Matter (NOM). Two methods, viz. efficient microwave combustion (ECM) and hydroxide mediated approach (HMA), were used to synthesize CONPs. The developed materials were characterized by the field emission scanning electron microscope (FESEM) with energy dispersive X-ray (EDX) and the Fourier transform infrared spectroscopy (FTIR). Moreover, the X-ray powder diffraction (XRD) confirmed the cubic structure with an average crystal size of 20.16 nm (CONP-I) and 6.75 nm (CONP-II). The observed point of zero (pHPZC) charge was approximately 7.0. The enhanced BET surface area (85.43 m2/g, 78.59 m2/g) and pore volume (0.007310 cm3/g, 0.006761 cm3/g) of CONPs support the higher adsorption. The effect of operational parameters (pH, contact time, and adsorbent dosage) and thermodynamical aspects of adsorption was also investigated. The Temkin isotherms described the experimental data better, with a maximum adsorption capacity of 238.9 mg/g (CONP-I) at neutral pH. Further, the experimental data can better be modeled by the pseudo-second-order kinetics (R2, 0.9851). Overall, CONPs possess great efficiency for the simultaneous removal of DOC (94%), UV254 (93%), adsorption slop index (ASI) (95%), phenolic content (88%), and carboxylic content (73%).






Similar content being viewed by others
Data availability
Data collected and analyzed in this study are available from the corresponding author upon request.
References
Adusei-Gyamfi J, Ouddane B, Rietveld L, Cornard JP, Criquet J (2019) Natural organic matter-cations complexation and its impact on water treatment: a critical review. Water Res 160:130–147
Baghoth SA (2012) Characterizing natural organic matter in drinking water treatment processes and trains. IHE Delft Institute for Water Education
Cates RG, Rhoades DF (1977) Patterns in the production of antiherbivore chemical defenses in plant communities. Biochem Syst Ecol 5(3):185–193
Chelliah M, Rayappan JBB, Krishnan UM (2012) Synthesis and characterization of cerium oxide nanoparticles by hydroxide mediated approach. JApSc 12(16):1734–1737
Chen J, Gu B, LeBoeuf EJ, Pan H, Dai S (2002) Spectroscopic characterization of the structural and functional properties of natural organic matter fractions. Chemosphere 48(1):59–68
Davis JA (1982) Adsorption of natural dissolved organic matter at the oxide/water interface. Geochim Cosmochim Acta 46(11):2381–2393
Farahmandjou M, Zarinkamar M, Firoozabadi TP (2016) Synthesis of Cerium Oxide (CeO2) nanoparticles using simple CO-precipitation method. Revista Mexicana de Física 62(5):496–499
Garg VK, Kumar R, Gupta R (2004) Removal of malachite green dye from aqueous solution by adsorption using agro-industry waste: a case study of Prosopis cineraria. Dyes Pigments 62(1):1–10
Hayes MH, MacCarthy P, Malcolm RL, Swift RS (1989) Humic substances II. Search Struct:4–31
Kim HC, Yu MJ (2005) Characterization of natural organic matter in conventional water treatment processes for selection of treatment processes focused on DBPs control. Water Res 39(19):4779–4789
Korshin G, Chow CW, Fabris R, Drikas M (2009) Absorbance spectroscopy-based examination of effects of coagulation on the reactivity of fractions of natural organic matter with varying apparent molecular weights. Water Res 43(6):1541–1548
Krzeminski P, Vogelsang C, Meyn T, Kohler SJ, Poutanen H, de Wit HA, Uhl W (2019) Natural organic matter fractions and their removal in full-scale drinking water treatment under cold climate conditions in Nordic capitals. J Environ Manag 241:427–438
Kumari M, Gupta SK (2018) Removal of aromatic and hydrophobic fractions of natural organic matter (NOM) using surfactant modified magnetic nanoadsorbents (MNPs). Environ Sci Pollut Res 25(25):25565–25579
Li R, Li Q, Gao S, Shang JK (2012) Exceptional arsenic adsorption performance of hydrous cerium oxide nanoparticles: part A. Adsorption capacity and mechanism. Chem Eng J 185:127–135
Mahato JK, Gupta SK (2020) Modification of Bael fruit shell and its application towards natural organic matter removal with special reference to predictive modeling and control of THMs in drinking water supplies. Environ Technol Innov 18:100666
Matilainen A, Gjessing ET, Lahtinen T, Hed L, Bhatnagar A, Sillanpaa M (2011) An overview of the methods used in the characterization of natural organic matter (NOM) in relation to drinking water treatment. Chemosphere 83(11):1431–1442
Naghizadeh A, Nasseri S, Rashidi AM, Rezaei Kalantary R, Nabizadeh R, Mahvi AH (2013) Adsorption kinetics and thermodynamics of hydrophobic natural organic matter (NOM) removal from aqueous solution by multi-wall carbon nanotubes. Water Sci Technol Water Supply 13(2):273–285
Nomura J, Imai H, Miyake T (1990) Removal of fluoride ion from wastewater by a hydrous cerium oxide adsorbent. Emerging Technologies in Hazardous Waste Management 157–172
O’Driscoll C, McGillicuddy E, Croot P, Bartley P, McMyler J, Sheahan J, Morrison L (2020) Tracing sources of natural organic matter, trihalomethanes and metals in groundwater from a karst region. Environ Sci Pollut Res:1–14
Oladipo AA, Ahaka EO, Gazi M (2019) High adsorptive potential of calcined magnetic biochar derived from banana peels for Cu 2+, Hg 2+, and Zn 2+ ions removal in single and ternary systems. Environ Sci Pollut Res 26(31):31887–31899
Oladoja NA, Seifert ML, Drewes JE, Helmreich B (2017) Influence of organic load on the defluoridation efficiency of nano-magnesium oxide in groundwater. Sep Purif Technol 174:116–125
Omri A, Benzina M, Trabelsi W, Ammar N (2014) Adsorptive removal of humic acid on activated carbon prepared from almond shell: approach for the treatment of industrial phosphoric acid solution. Desalin Water Treat 52(10-12):2241–2252
Priya T, Mohanta VL, Mishra BK (2017) Performance evaluation of zirconium oxychloride for reduction of hydrophobic fractions of natural organic matter. Sep Purif Technol 174:104–108
Priya T, Tarafdar A, Gupta B, Mishra BK (2018) Effect of bioflocculants on the coagulation activity of alum for removal of trihalomethane precursors from low turbid water. J Environ Sci 70:1–10
Rahman MS, Whalen M, Gagnon GA (2013) Adsorption of dissolved organic matter (DOM) onto the synthetic iron pipe corrosion scales (goethite and magnetite): effect of pH. Chem Eng J 234:149–157
Raichur AM, Basu MJ (2001) Adsorption of fluoride onto mixed rare earth oxides. Sep Purif Technol 24(1-2):121–127
Recillas S, Colón J, Casals E, González E, Puntes V, Sánchez A, Font X (2010) Chromium VI adsorption on cerium oxide nanoparticles and morphology changes during the process. J Hazard Mater 184(1-3):425–431
Saliu TD, Akinyeye OJ, Ololade IA, Unuabonah EI, Oladoja NA (2019) Expounding the role of interference on the recovery of nutrient fractions from aqua matrix using calcined gastropod shell. J Water Process Eng 27:152–161
Santschi PH, Xu C, Zhang S, Schwehr KA, Lin P, Yeager CM, Kaplan DI (2017) Recent advances in the detection of specific natural organic compounds as carriers for radionuclides in soil and water environments, with examples of radioiodine and plutonium. J Environ Radioact 171:226–233
Sillanpaa M, Ncibi MC, Matilainen A, Vepsalainen M (2018) Removal of natural organic matter in drinking water treatment by coagulation: a comprehensive review. Chemosphere 190:54–71
Skandari S, Torabian A, Nabi Bidhendi G, Baghdadi M, Aminzadeh B (2016) Preparation of engineered carbon nanotube materials and its application in water treatment for removal of hydrophobic natural organic matter (NOM). Desalin Water Treat 57(52):24855–24866
Tamizhdurai P, Sakthinathan S, Chen SM, Shanthi K, Sivasanker S, Sangeetha P (2017) Environmentally friendly synthesis of CeO 2 nanoparticles for the catalytic oxidation of benzyl alcohol to benzaldehyde and selective detection of nitrite. Sci Rep 7:46372
Teixeira MR, Rosa SM, Sousa V (2011) Natural organic matter and disinfection by-products formation potential in water treatment. Water Resour Manag 25(12):3005–3015
Wang J, Tian H, Ji Y (2015) Adsorption behavior and mechanism of humic acid on aminated magnetic nanoadsorbent. Sep Sci Technol 50(9):1285–1293
Wei L, Wang K, Zhao Q, Xie C, Qiu W, Jia T (2011) Kinetics and equilibrium of adsorption of dissolved organic matter fractions from secondary effluent by fly ash. J Environ Sci 23(7):1057–1065
Xiao H, Ai Z, Zhang L (2009) Nonaqueous sol−gel synthesized hierarchical CeO2 nanocrystal microspheres as novel adsorbents for wastewater treatment. J Phys Chem C 113(38):16625–16630
Yu L, Ma Y, Ong CN, Xie J, Liu Y (2015) Rapid adsorption removal of arsenate by hydrous cerium oxide–graphene composite. RSC Adv 5(80):64983–64990
Yu Y, Zhang C, Yang L, Chen JP (2017) Cerium oxide modified activated carbon as an efficient and effective adsorbent for rapid uptake of arsenate and arsenite: material development and study of performance and mechanisms. Chem Eng J 315:630–638
Zulfikar MA, Suri FI, Rusnadi, Setiyanto H, Mufti N, Ledyastuti M, Wahyuningrum D (2016) Fe3O4 nano-particles prepared by co-precipitation method using local sands as a raw material and their application for humic acid removal. Int J Environ Stud 73(1):79–94
Acknowledgments
The authors acknowledge the Department of Environmental Science and Engineering, Indian Institute of Technology (ISM), Dhanbad, India, to support the research work.
Author information
Authors and Affiliations
Contributions
Jaydev Kumar Mahato (first): investigation and writing—original manuscript. Sunil Kumar Gupta (Corresponding author): conceptualization and supervision.
Corresponding author
Ethics declarations
Ethics Approval
No ethical (human or animal) approval was required to conduct the study.
Consent to Participate
Not applicable.
Consent for publication
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 242 kb)
Rights and permissions
About this article
Cite this article
Mahato, J.K., Gupta, S.K. Exceptional adsorption of different spectral indices of natural organic matter (NOM) by using cerium oxide nanoparticles (CONPs). Environ Sci Pollut Res 28, 45496–45505 (2021). https://doi.org/10.1007/s11356-021-13964-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-13964-w