Abstract
The outbreak of novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has affected the entire world with its infectious spread and mortality rate. The severe cases of coronavirus disease 2019 (COVID-19) are characterized by hypoxia and acute respiratory distress syndrome. In the absence of any specific treatment, just the preventive and supportive care options are available. Therefore, much focus is given to assess the available therapeutic options not only to avoid acute respiratory failure and hypoxia but also to reduce the viral load to control the severity of the disease. The antimalarial drug hydroxychloroquine (HCQ) is among the much-discussed drugs for the treatment and management of COVID-19 patients. This article reviews the therapeutic potential of HCQ in the treatment of COVID-19 based on the available in vitro and clinical evidence, current status of registered HCQ-based clinical trials investigating therapeutic options for COVID-19, and environmental implications of HCQ.
Similar content being viewed by others
Introduction
Coronavirus disease 2019 (COVID-19) caused by a novel beta-coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has by far affected almost all countries. At the time of writing, there have been about 30,00000 confirmed cases and 200,000 deaths worldwide. Since December 2019, it started from Wuhan city, China (Huang et al. 2020), and the nature of its spread worldwide seems to be exponential. It is an acute respiratory infectious disease (Ksiazek et al. 2003; Hasana et al. 2021) that spreads through droplets generated by coughing and sneezing, discharge from the nose, saliva, or by coming to the close contact of an infected person with incubation times of 2–14 days (Zhai et al. 2020).
The majority of patients are experiencing mild respiratory issues and patients with pre-existing chronic diseases are experiencing severe infection (Zaki et al. 2012; Menachery et al. 2015). Besides, COVID-19 patients have a high risk of dying due to large incidences of bacterial superinfection and septic shock. Despite the hasty global spread of the COVID-19 infection, there is no reliable clinical data yet to endorse any specific therapeutics (Surti et al. 2020). Therefore, symptomatic, organ supportive treatment including respiratory therapy in acute pulmonary failure is recommended by doctors worldwide.
Although various countries are adopting a series of preventive measures, the pandemic is difficult to be contained due to a lack of specific medicines which is evident from the fact that the number of patients of COVID-19 is on the rise (Wang et al. 2020a; Şimşek Yavuz and Ünal 2020; Kumar et al. 2020; Kabir et al. 2020). Special attention is given for the prevention, mitigation, and management of SARS-CoV-2-infected patients due to the absence of specific antiviral therapeutics (Behl et al. 2020; Jean et al. 2020; Siddiqui et al. 2020a). Therefore, COVID-19 therapy is so far is supportive. The vaccines against COVID-19 will probably take at least a year to become available, and the chemoprophylaxis is the only option in hand to treat infected patients. During this supportive management, hydroxychloroquine (HCQ) is being widely used (Ferner and Aronson 2020; Touret and de Lamballerie 2020; Yazdany and Kim 2020; Sinha and Balayla 2020; Mack 2020; Colson et al. 2020; Sahraei et al. 2020; Singh et al. 2020; Agrawal et al. 2020; Guastalegname and Vallone 2020).
Mechanism of action of hydroxychloroquine
HCQ is a hydroxy analog of chloroquine and commonly known as plaquenil. HCQ has N-hydroxyethyl in place of the N-diethyl side chain in the chloroquine moiety (Fig. 1) and probably due to this structural difference, it is more soluble and less toxic than chloroquine (Lui et al. 2020). HCQ is widely used in autoimmune and malarial diseases. It is reported to be a potent broad-spectrum antiviral agent (Savarino et al. 2006; Yan et al. 2013; Rolain et al. 2007). HCQ is absorbed from the upper intestinal tract upon oral administration. Its bioavailability ranges between 0.7 and 0.8. HCQ metabolism in liver and renal clearance has been reported to be 16% and 21% respectively (Schrezenmeier and Dörner 2020). HCQ is reported to have the in vitro anti-SARS-Co-2 activity (Yao et al. 2020; Biot et al. 2006; Vincent et al. 2005).
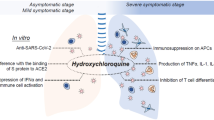
The virus requires slightly acidic pH in the endosomes to enter the cells. HCQ increases the pH in the endosomes thereby, stops their acidification and maturation, and interferes with the entry of the virus in the cell (Fig. 2). This seems to be the principal mechanism of action of HCQ (Siddiqui et al. 2020b; Macfarlane and Manzel 1998; Hacker et al. 1998).
a The spherical, coronaviruses with RNA genome (single-stranded). The genome encodes 4 main structural proteins: spike (S), envelope (E), nucleocapsid (N), and membrane (M) proteins. S contains two subunits S1 for receptor binding and S2 for membrane fusion. b HCQ increases pH in the endosomes thereby, stops their acidification and maturation, and interferes with the entry of the virus in the cell
The HCQ also inhibits the Toll-like receptor (TLR) and TLR-driven signal transduction. The alteration in pH of endosome (involved in the TLR processing) and/or inhibiting TLR7 and TLR9 from binding their respective ligands (RNA and DNA) downregulates TLR signaling and thereby reduces the formation of pro-inflammatory cytokines (Fig. 3), including the interleukin-6 production and imparts modulating effects on activated immune cells (Jorge et al. 2018; Sahraei et al. 2020; Wu et al. 2020).
Although, the antiviral activity of chloroquine and HCQ is similar theoretically (Tan et al. 2018). However, the major advantage of HCQ is its lesser adverse effects and better compatibility with other antiviral agents such as lopinavir, ritonavir, ribavirin, oseltamivir, and remdesivir as compared to chloroquine in the treatment of COVID-19 patients (Wang et al. 2020b). Also, HCQ does not interfere with intravenous immunoglobulins and interferons. The chloroquine/hydroxychloroquine do not influence the interferon (IFN) pathways; however, some are positively regulated by the drugs, others are negatively regulated (Nallar and Kalvakolanu 2014; Lee and Ashkar 2018). Another advantage of HCQ is its wide availability in some countries in comparison to chloroquine. Due to the potential chemoprophylaxis and therapeutic promises of HCQ with the abovementioned therapeutics, a large number of clinical trials are under investigation.
Current status of hydroxychloroquine in COVID-19 treatment
HCQ and chloroquine are both not FDA-approved for the treatment of COVID-19 because the safety profile of these agents has only been investigated for FDA approved indications and not COVID-19. One of the recent in vitro studies to compare the therapeutic activity of HCQ and chloroquine in the SARS-CoV-2 infected Vero cells reported that HCQ is more effective (EC50=0.72 μM) in comparison to chloroquine (EC50=5.47 μM). Also, the study demonstrated that HCQ, at 400 mg given twice a day (loading dose) and 200 mg twice a day for subsequent 4 days (maintenance dose) as compared to the chloroquine given 500 mg twice for 5 day, shows three times better potency against SARS-CoV-2 in physiologically based pharmacokinetic model (Yao et al. 2020). The study suggests that the therapeutic activity and safety profile of the HCQ is better than the chloroquine. The study’s findings seem to be important for the selection of more potent drugs from the available aminoquinolines during the treatment of COVID-19 patients. This in vitro study seems to just indicative, and there is every possibility that these measured EC50 values differ while performing in vivo investigations. The results of the limited clinical trials available to date for assessing the effect of HCQ on COVID-19 patients could be analyzed in two categories: clinical results supporting the HCQ therapy and those against it.
Clinical evidence supporting the hydroxychloroquine therapeutic activity for COVID-19 patients
An open-label non-randomized clinical trial (EU clinical trials register no. 2020-000890-25/FR; N=36; 20 HCQ-treated, 16 control) reported that 70% of the HCQ-treated SARS-CoV-2-infected patients (200 mg thrice a day) were able to get cured of the virus as compared to the 12.5% in the control group after 6 days. This study also reported that HQC has synergistic effects with azithromycin in COVID-19 patients as 100% of the patients receiving the HCQ-azithromycin combination get shredded the virus in 6 days (Gautret et al. 2020). This study indicates that HCQ is an efficient drug in reducing the viral load from COVID-19 patients. However, the sample size of the study is small as a result, and there is every chance that mild adverse reactions of HCQ will not be detected. Also, the reason behind HCQ-non-respondence in the remaining patients is not clear.
In a parallel-group randomized trial (ChiCTR2000029559), 62 non-sever hospitalized COVID-19 patients (31 HCQ treated; 31 control with standard therapy) were included to study the efficacy of HCQ in the treatment. Among the 31 HCQ-treated patients (400 mg/day), 25 showed better improvement as compared to 17 out of 31 from the control group after 5 days of treatment. There was a significantly shorter time to clinical recovery, fever recovery (2.2 days compared to 3.2 days), cough reduction (2 days as compared to 3.1 days) reported in the HCQ-treated patient. All the patients progressed to the severe SARS-CoV-2 infection (4 out of 62) were from the control group (Chen et al. 2020a). The study confirms the therapeutic promises of HCQ in non-severe hospitalized COVID patients. However, this trial is not peer-reviewed and might contain discrepancies such as patient self-reported cough-remission, differences between registered trial, and reported results unavailability of enough data to replicate the reported findings, not properly blinded study, etc. Even if we partially rely on this study, the effectiveness of HCQ in severely ill patients is still under question.
Another non-peer-reviewed trial to find out the association between HCQ-treatment and mortality of the hospitalized and critically ill COVID-19 patients claims to find reduced mortality in the HCQ-treated patients (Yu et al. 2020). A total of 568 critically ill COVID-19 patients were considered in the study where 48 of them received 200 mg of HCQ twice/day along with the basic therapy, and the remaining 520 were taken as control (supported by basic therapy only). The mortality in HCQ-treated patients was reported to be 18.8% as compared to that of 45.8% for the control group. The study also claims that HCQ significantly attenuates the cytokine storm in COVID-19 patients. This study recommends the HCQ treatment for severely ill patients. However, the reasons for the high mortality rate in the control group were not explained.
Clinical evidence against the therapeutic activity of hydroxychloroquine for COVID-19 patients
A pilot study report (NCT04261517; N=30; 15 HCQ treated, 15 Control) suggests that there is not much difference in the HCQ treated group (dose 400 mg/day for 5 days) and control group (standard treatment), COVID-19 patients. The nucleic acid in the throat swabs found negative in 86.7% HCQ-treated patients, whereas in the control group, it was found to be 93.3% (Chen et al. 2020b). The study seems to be open, and not much is there to learn, but it casts doubts on the effectiveness of HCQ in COVID-19.
A prospective review was performed to evaluate the clinical efficiency of HCQ (600 mg/day for 10 days) with azithromycin (500 mg/day on day 1; 250 mg/day for the next 2–5 days) in 11 severely SARS-CoV-2-infected hospitalized patients. Two patients were shifted to the intensive care unit within 5 days of treatment, 1 patient died, and for 1 patient, the therapy was discontinued due to prolongation of QT. Besides, 8 out of 10 remaining patients were SARS-CoV-2 positive (nasopharyngeal swabs tested by PCR) even after 5–6 days of HCQ-azithromycin combination treatment (Molina et al. 2020). This study indicates that for severely ill patients, HCQ may not be as effective as non-severe hospitalized COVID-19 patients. Its effectiveness may depend upon the patients’ condition, such as associated comorbidities and severity of SARS-CoV-2 infection.
Another observational study was done by Geleris and colleagues (Geleris et al. 2020) to find an association between HCQ and hospitalized COVID-19 patients’ outcomes. The investigators studied 1376 consecutive patients who were admitted between March 7 and April 8, 2020, to New York City medical center. Patients with less than 94% resting oxygen saturation were considered for the HCQ treatment based on clinicians’ discretion. A total of 811 patients were given HCQ (loading dose 600 mg twice on day 1 and maintenance dose of 400 mg per day for 5 median days), among which 60% of such patients also received azithromycin (500 mg loading dose and 250 mg maintenance dose) for the total median duration of 22.5 days. The study concludes that there was no substantial connection between the use of HCQ and intubation or death (hazard ratio, 1.04, 95% confidence interval, 0.82 to 1.32) (Geleris et al. 2020). The study indicates that the death/intubation rate was about twice in the HCQ-treated patients as compared to the patients who did not receive the drug. While the study results are consistent with all the sensitivity analysis, the major concern with this observational study is the significant differences in the clinical conditions between the HCQ-treated and untreated patients. Patients treated with HCQ were significantly ill at baseline than the untreated groups. Therefore, the outcome of the result of this control-devoid study may be biased against the HCQ.
In another observational study, Rosenberg and co-workers (Rosenberg et al. 2020) investigated the therapeutic efficacy of HCQ, azithromycin, and their combination to assess the mortality rate of 1438 hospitalized COVID-19 patients. These patients were randomly selected from the hospitals in the New York City metropolitan region. The selected patients were divided into four groups of 735, 271, 211, and, 221 patients who received HCQ+azithromycin, HCQ alone, azithromycin alone, and neither drug, respectively. The death probabilities of the patients with HCQ+azithromycin, HCQ alone, azithromycin alone, and neither drug were reported to be 25.7%, 19.9%, 10%, and 12.7% respectively. The adjusted hazard ratios as compared to the patients who received neither drugs were reported to be 1.35 for HCQ+azithromycin, 1.08 for HCQ alone, and 0.56 for the azithromycin alone. The study concluded that there were no notable differences in mortality of the hospitalized COVID-19 patients with or without treatment of HCQ+azithromycin, HCQ alone, and azithromycin alone (Rosenberg et al. 2020). The patients’ receiving drug therapy had pre-existing diabetes, abnormal chest imaging findings, respiratory rate >22/min, oxygen saturation less than 90%, and aspartate aminotransferase were higher than 40 U/L. The study seems to be biased as the patients who received the drugs for treatment were significantly ill at the baseline and had multiple other risk factors as compared to the ones received neither drugs. But after receiving treatment had about the same mortality rates were reported with the much sicker patients who received the treatment and those patients with a milder course of the illness and fewer risk factors. However, the study concluded that there were no significant benefits due to the treatment.
In the latest studies, Cavalcanti et al. (2020) performed Open-label, randomized, controlled trial with 667 patients. These patients were randomly divided into three groups with 1:1:1 ratio. The first group was subjected to the standard therapy, the second group got 400 mg HCQ twice a day, and the third group received 400 mg azithromycin once a day for 7 days in addition to the HCQ. The clinical status of these patients was analyzed after 15 days of treatments and no significant improvements were observed. Rather, increased frequency of QTc interval prolongation was observed in patients who received azithromycin and/or HCQ as compared to those received standard therapy for COVID-19 (Cavalcanti et al. 2020). Although this study has numerous limitations such as non-blinded trial, median time of onset of symptoms, and small sample size. However, it is also important to note that this study raises serious doubts about the therapeutic efficacy of HCQ and chloroquine with or without azithromycin. The clinical trial details in the favor and against therapeutic efficacy of HCQ in COVID-19 are summarized in Tables 1 and 2 respectively.
In a randomized clinical trial, promises of prophylaxis abilities of HCQ in COVID-19 infected patients were assessed (Boulware et al. 2020). The trial was randomized, double-blind, and placebo-controlled to evaluate whether it can prevent symptomatic infection after the viral infection. The study was conducted on 821 asymptomatic patients from the USA and Canada. The study finds that even after the treatment with HCQ, given to the patients after 4 days of SARS-CoV-2 infection did not show any significant difference as compared to the placebo-treated patients. The dose that was applied to these patients was 800 mg (loading dose) followed by 600 mg and then 600 mg a day for next 4 days. The study concludes that the HCQ did not show any significant effect when used for post-exposure prophylaxis.
While HCQ at present is widely used all around the world for the treatment of COVID-19 patients, the clinical data for convincing therapeutic evidence for COVID-19 patients are still missing. The results of the small clinical studies are uncontrolled and, in some cases, poorly reported. As of now, the evidence in hand for therapeutic efficacy of HCQ in COVID-19 is thin and mixed therefore, more robust and conclusive clinical findings are warranted to understand the effectiveness of HCQ. Nevertheless, based on limited in vitro and available conditional clinical data, HCQ sulfate and chloroquine phosphate are currently being recommended and used for hospitalized COVID-19 patients in many countries. An interesting study suggests that there was a significant increase in number of prescriptions of HCQ in the US from October 2019 to March 2020 (Shehab et al. 2020). The importance HCQ evaluation for COVID-19 is evident from the fact that it has the highest number of active clinical trials among all the available drug therapies for the management of COVID-19 (Phase 1: NCT04333654; Phase 2: NCT04333225, NCT04329923; Phase 3: NCT04329611, NCT04340544, NCT04334148, etc.; Phase 4: ChiCTR2000029868).
Many other therapeutic options have moved to the clinical trials including HCQ combined with azithromycin (NCT04322396, NCT04341207, NCT04335552), lopinavir and ritonavir (NCT04328285, NCT04307693), sarilumab/baricitinib (NCT04321993), ciclesonide (NCT04330586), remdesivir (NCT04321616), tocilizumab (NCT04332094), etc. Table 1 depicts the ongoing clinical trial status of HCQ alone and in-combinations with various antimicrobials and adjunct drugs for the management of COVID-19. Recently, FDA has also authorized the use of HCQ sulfate and chloroquine phosphate during COVID-19 pandemic in the prophylaxis and treatment of patients for which clinical trial is not existing or involvement is not possible, under section 564(c) of the Federal Food, Drug, and Cosmetic Act. This authorization has three conditions to follow; one, FDA-approved HCQ sulfate and chloroquine phosphate for other uses and with FDA-approved labeling and authorized fact sheets to be used; second, both drugs must be administered by healthcare pursuant upon the valid prescription of a licensed practitioner; third, these drugs may only be used for the treatment of adult and adolescent patients with the weight of 50 kg or above, hospitalized with COVID-19. WHO temporarily halted the HCQ wing of SOLIDARITY trials to review the HCQ/chloroquine trial’s data available to date by the safety and monitoring board. This was done in response to patient safety concerns raised by some of the studies. However, these studies could not satisfy the claims mentioned in their study upon second phase validation, and WHO restarted the HCQ wing of trials again. The rest of the three wings of the SOLIDARITY trails, ramdesivir, lopinavir/ritonavir with or without interferon β1a, are continued without any halt. The chloroquine is a recommended treatment for Covid-19 in the new national Chinese guidelines. The adult dose of 500 mg chloroquine twice per day for not more than 10 days is included in the 6th version of the COVID-19 treatment guidelines by the National Health Commission of the People’s Republic of China (National Health Commission of the People’s Republic of China 2020). The Indian Council of Medical Research (highest scientific body of the country) has recommended chemoprophylaxis with HCQ (400 mg twice on day 1, then 400 mg once a week after that) for asymptomatic household contacts of confirmed patients and asymptomatic healthcare workers involved in the treatment of patients with suspected/confirmed COVID-19 (D'Cruz 2020). The prophylactic HCQ is targeted to individuals at high risk rather than the general population, and approach seems prudent in the state of the first spread of a new virus where data paucity is expected (Rathi et al. 2020; Rubin et al. 2020).
Doses and side effects of hydroxychloroquine
The HCQ must be given orally. The suggested dose for the treatment of COVID-19 hospitalized patients who weigh 50 kg or above is 800 mg on the first day of treatment and then based on clinical evaluation 400 mg daily for 4–7 days. The recommended dose and duration may further be modified as the clinical trial data is available. The dosage of HCQ sulfate is often presented as an equivalent HCQ base (each 200 mg tablet of HCQ sulfate corresponds to 155 mg base) (FDA 2020). Noteworthily, its optimal dose and treatment duration for COVID-19 are unknown.
The common side effects of the HCQ are nausea, stomachache, vomiting, headache, and in some cases itching. These minor side effects do not require the discontinuation of the treatment and could be reduced by taking the HCQ with food. However, some patients could have major side effects on the prolonged use of HCQ, such as irregular heartbeats, convulsions or seizures, eyes yellowing, retinopathy, hazy vision, hearing issues, muscle stiffness, and bruising of the skin (Ling Ngan Wong et al. 2008; Marmor et al. 2011; McChesney 1983; Popert 1976). The small prospective studies have shown that there is no increase in spontaneous abortion or birth defect rate upon HCQ treatment during pregnancy.
Drug interactions of hydroxychloroquine
Antacids and kaolin reduce the absorption of HCQ (Khalil 1977; McElnay et al. 1982). Therefore, the time interval should be a minimum of 4 h between HCQ and intake of these agents. Cimetidine is a CYP450 pan-inhibitor and co-administration results in possible increases in HCQ levels (Furst 1996) cardiac glycosides such as digoxin are increased in the serum in the presence of HCQ and serum concentration of digoxin should be thoroughly monitored while the HCQ treatment (Leden 1982). Similarly, HCQ may increase the effects of insulin and other antidiabetic drugs (Wondafrash et al. 2020). In this case, a dose reduction of antidiabetics is suggested. With arrhythmogenic agents such as moxifloxacin, amiodarone, and azithromycin, HCQ enhances the risk of ventricular arrhythmias induction (Somer et al. 2000).
Therefore, the time-gap between the HCQ and such arrhythmogenic drug administration should be adapted. The co-administration of HCQ with ampicillin should also be avoided because the bioavailability of ampicillin is reduced significantly by HCQ (Stokkermans and Trichonas 2020). On the cometary, HCQ increases the serum concentration of cyclosporine (Nampoory et al. 1992). The anti-cancerous agents, such as tamoxifen with HCQ induces retinal toxicity, thus concomitant administration of these drugs should be avoided. Apart from these, HCQ co-administration with antimalarial drug mefloquine increases the risk of convulsions (Golden et al. 2015). The HCQ is a substrate of CYP2C8, CYP2D6, and CYP3A4/5, and has been proven to elevate metoprolol levels via CYP2D6 inhibition (Kim et al. 2003; Kumar et al. 2016). Therefore, it is extremely vital to assess and review the benefit and risk balance before prescribing HCQ in such cases.
Environmental implications of hydroxychloroquine
There is a sudden rise in demand for HCQ in the global market which is inspired by the preliminary reports on its effectiveness in the treatment of COVID-19, resulting in its production upscale. High manufacturing volumes of HCQ have the potential for being environment persistent and bio-accumulative. HCQ undergoes a series of biotransformation upon administration and eliminated both in conjugated and unconjugated form through urine and fecal matter. During production, the wastewater from the pharmaceutical plats must undergo a series of treatments failing to which the drug could enter the aquatic environment. HCQ has a serious chronic threat to the aquatic environment as the drug belongs to a group of quinolone derivatives, which is recalcitrant, toxic, persistent, teratogenic, and carcinogenic for the aquatic organisms. The behavior and fate of pharmaceutical HCQ in the aquatic environment significantly unexplored. The photodegraded products could impart significant toxicity, and sometimes more detrimental to the aquatic environment as compared to the parent molecule (Ferraz et al. 2014; Kawabata et al. 2013). One of the recent studies demonstrated that the chloroquine phosphate and HCQ kill the hair cells in the zebrafish lateral line when used in varying concentrations for 1–24 h (Davis et al. 2020). Chloroquine derivatives are used in controlling protozoan parasites and have some use in eradicating certain metazoan fish parasites and skin diseases in small concentrations. However, acute and sublethal concentrations of chloroquine may pose histological and enzymological alterations in aquarium fish Cyprinus carpio. The acute toxicity may appear due to high doses and the sublethal dose may accumulate and remain active in the aquarium to cause side effects (Ramesh et al. 2017). These preliminary studies indicate about the vulnerability of aquatic organisms towards chloroquine and its derivatives.
The environmental persistence of HCQ has recently been investigated and the preliminary findings confirmed that HCQ undergoes photodegradation in the aquatic environment (Dabic et al. 2019). The study revealed that the photolysis of HCQ is pH sensitive. At pH 9 and 7, the photolytic degradation of HCQ was reported to be 40 min and 22 h respectively. However, at pH 4, the HCQ was not degraded even after 52 h. The quantum yield of the HCQ is also pH-dependent and increases with the protonation. This indicates that in an acidic environment (e.g., pH=4), HCQ has limited photosensitivity for direct degradation and its photosensitizers may have a vital role in indirect photodegradation of HCQ in the aquatic environment (Gonc̀alves et al. 2011). Besides, the presence of sulfate, chloride, and bromide decreases the extent of photolysis of HCQ. One of HCQ metabolites in humans is desethyl-HCQ, which is also the photodegradation product is expected to be more environmentally persistent. Moreover, the majority of photodegraded products of HCQ presumably has more hydrophilicity and polarity (due to hydroxyl group addition). Therefore, such products are more persistent in the aquatic environment as they are less susceptible to sediment or soil-sorption. Nevertheless, the uses of HCQ have been assessed to pose a negligible risk of environmental impact. HCQ is potentially persistent as it shows 0% biotic degradation in 28 days (Sanofi internal report 2016). Partitioning coefficient (Log D) of HCQ at pH 7.4 is reported to be 0.62 (using Sirius GLpK automated computerized) which is indicative of low potential to bioaccumulate (Log D>4 indicates potential to bioaccumulation) (Warhurst et al. 2003). The overall assessment of HCQ towards its environmental safety seems to be satisfactory which is further substantiated by ecotoxicological studies (Table 2) suggesting that HCQ and its sulfate derivative pose “no effect concentration” in aquatic organisms. However, some of the studies indicate the chloroquine and its derivatives as emerging pollutants (Daughton 2014; Zurita et al. 2005) especially when wastewater treatment systems are inadequate to process such drugs (Ashfaq et al. 2017). The arthropods are most vulnerable to environmental pollution. One of the recent studies on the effects of HCQ and chloroquine on ants suggests that both drugs affect food consumption, cognition, and social relationships. In addition to these side effects, audacity, adaptation to adverse conditions, and tactile perception of the ants are also negatively affected. The cognition traits of ants were found to be more vulnerable with chloroquine and the muscle functioning traits were more affected by HCQ. Although, there was no dependence reported in the study (Cammaerts and Cammaerts 2020).
Conclusion
As acidification is vital for the viral entry as well as endosome growth and function, the HCQ seems to be crucial to the inhibition of the viral entry to the cells. It increases the pH of endosomes/lysosomes; however, its impact on the endosomal/lysosomal morphology and pH value is still under investigation. Besides, clinical investigations have detected the high levels of cytokines in the plasma of severely sick individuals infected with SARS-CoV-2, which suggests that cytokine concentration is linked with the severity of illness. HCQ has been effectively used in auto-immune diseases as a safe anti-inflammatory agent and it may decrease the pro-inflammatory cytokine expression in COVID-19 as per preliminary studies. However, whether it is effective especially when the lungs are distressfully inflamed to expel the SARS-CoV-2 virus due to its hyperactive immune system in ICU patients is clinically unproven yet. The available clinical evidence seems lean, mixed, and insufficient to produce any conclusive clinical evidence for the effectiveness of HCQ in severely ill COVID-19 patients. Nevertheless, based on available in vitro and some clinical studies, HCQ seems to be promising for mildly infected patients until the availability of conclusive clinical investigations to clarify its precise role (if any). Besides, there are no environmental concerns about the use of HCQ reported so far.
Data availability
Not applicable.
References
Adedeji AO, Sarafianos SG (2014) Antiviral drugs specific for coronaviruses in preclinical development. Curr Opin Virol 8:45–53. https://doi.org/10.1016/j.coviro.2014.06.002
Agostini ML, Andres EL, Sims AC, Graham RL, Sheahan TP, Lu X et al (2018) Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio 9(2):1–15. https://doi.org/10.1128/mBio.00221-18
Agrawal S, Goel AD, Gupta N (2020) Emerging prophylaxis strategies against COVID-19. Monaldi Arch Chest Dis 90(1). https://doi.org/10.4081/monaldi.2020.1289
Amsden GW (2005) Anti-inflammatory effects of macrolides - an underappreciated benefit in the treatment of community-acquired respiratory tract infections and chronic inflammatory pulmonary conditions? J Antimicrob Chemother 55(1):10–21. https://doi.org/10.1093/jac/dkh519
Ashfaq M, Nawaz Khan K, Saif Ur Rehman M, Mustafa G, Faizan Nazar M, Sun Q, Iqbal J, Mulla SI, Yu CP (2017) Ecological risk assessment of pharmaceuticals in the receiving environment of pharmaceutical wastewater in Pakistan. Ecotoxicol Environ Saf 136:31–39. https://doi.org/10.1016/j.ecoenv.2016.10.029
Behl T, Kaur I, Bungau S, Kumar A, Uddin MS, Kumar C, Pal G, Sahil, Shrivastava K, Zengin G, Arora S (2020) The dual impact of ACE2 in COVID-19 and ironical actions in geriatrics and pediatrics with possible therapeutic solutions. Life Sci 257:118075. https://doi.org/10.1016/j.lfs.2020.118075
Beigelman A, Mikols CL, Gunsten SP, Cannon CL, Brody SL, Walter MJ (2010) Azithromycin attenuates airway inflammation in a mouse model of viral bronchiolitis. Respir Res 11:90. https://doi.org/10.1186/1465-9921-11-90
Biot C, Daher W, Chavain N, Fandeur T, Khalife J, Dive D, De Clercq E (2006) Design and synthesis of hydroxyferroquine derivatives with antimalarial and antiviral activities. J Med Chem 49:2845–2849. https://doi.org/10.1021/jm0601856
Boulware DR, Pullen MF, Bangdiwala AS, Pastick KA, Lofgren SM, Okafor EC, Skipper CP, Nascene AA, Nicol MR, Abassi M, Engen NW, Cheng MP, LaBar D, Lother SA, MacKenzie LJ, Drobot G, Marten N, Zarychanski R, Kelly LE, Schwartz IS, McDonald EG, Rajasingham R, Lee TC, Hullsiek KH (2020) A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med 383(6):517–525. https://doi.org/10.1056/NEJMoa2016638
Brown AJ, Won JJ, Graham RL, Dinnon KH, Sims AC, Feng JY, Cihlar T, Denison MR et al (2019) Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antivir Res 169:1–10. https://doi.org/10.1016/j.antiviral.2019.104541
Cammaerts M, Cammaerts R (2020) Side effects of chloroquine and hydroxychloroquine examined on ants as models. EC Pharmacol Toxicol 8(11):57–82
Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VC, Avezum A, Damiani LP, Marcadenti A, Kawano-Dourado L, Lisboa T, Junqueira DLM, de Barros e Silva PGM, Tramujas L, Abreu-Silva EO, Laranjeira LN, Soares AT, Echenique LS, Pereira AJ, Freitas FGR, Gebara OCE, Dantas VCS, Furtado RHM, Milan EP, Golin NA, Cardoso FF, Maia IS, Hoffmann Filho CR, Kormann APM, Amazonas RB, Bocchi de Oliveira MF, Serpa-Neto A, Falavigna M, Lopes RD, Machado FR, Berwanger O (2020) Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N Engl J Med 383:2041–2052. https://doi.org/10.1056/NEJMoa2019014
Chen Z, Hu J, Zhang Z, Jiang S, Han S, Yan D, Zhuang R et al (2020a) Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. J Zhejiang Univ (Med Sci) 49(2):215–219. https://doi.org/10.1101/2020.03.22.20040758
Chen J, Liu D, Liu L, Liu P, Xu Q, Lu X, Ling Y, Huang D, Song S, Zhang D, Qian Z et al (2020b) A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (covid-19). J Zhejiang Univ (Med Sci) 49(1). https://doi.org/10.3785/j.issn.1008-9292.2020.03.03
Colson P, Rolain JM, Lagier JC, Brouqui P, Raoult D (2020) Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents 55(4):105932. https://doi.org/10.1016/j.ijantimicag.2020.105932
Dabic H, Babic S, Skoric I (2019) The role of photodegradation in the environmental fate of hydroxychloroquine. Chemosphere 230:268–277
Daughton CG (2014) The Matthew effect and widely prescribed pharmaceuticals lacking environmental monitoring: case study of an exposure-assessment vulnerability. Sci Total Environ 466-467:315–325. https://doi.org/10.1016/j.scitotenv.2013.06.111
Davis SN, Wu P, Camci ED, Simon JA, Rubel EW, Raible DW (2020) Chloroquine kills hair cells in zebrafish lateral line and murine cochlear cultures: implications for ototoxicity. Hear Res 395:108019. https://doi.org/10.1016/j.heares.2020.108019
D'Cruz M (2020) The ICMR bulletin on targeted hydroxychloroquine prophylaxis for Covid-19: need to interpret with caution. Indian J Med Ethics V(2):100–102. https://doi.org/10.20529/IJME.2020.040
de Wit E, Feldmann F, Cronin J, Jordan R, Okumura A, Thomas T, Scott D, Cihlar T, Feldmann H (2020) Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci U S A 117(12):6771–6776. https://doi.org/10.1073/pnas.1922083117
Deeks ED (2018) Bictegravir/emtricitabine/tenofovir alafenamide: a review in HIV-1 infection. Drugs 78(17):1817–1828. https://doi.org/10.1007/s40265-018-1010-7
Duan Y, Zhu HL, Zhou C (2020) Advance of promising targets and agents against COVID-19 in China. Drug Discov Today S1359-6446(20):30098–30102. https://doi.org/10.1016/j.drudis.2020.02.011
Fass (2020) Plaquenil®. Accessed: 15 November 2020. Available at: https://www.fass.se/LIF/product?-1.ILinkListener-documentTabPanel-tabs-panel-article~tools~top-articletools-printlink&userType=2&nplId=19610412000017&docType=78&docTypeDynTab=78
Favalli EG, Biggioggero M, Maioli G, Caporali R (2020) Baricitinib for COVID-19: a suitable treatment? Lancet Infect Dis S1473-3099(20):30262–30270. https://doi.org/10.1016/S1473-3099(20)30262-0
FDA (2020) Fact sheet for health care providers emergency use authorization (EUA) of hydroxychloroquine sulfate supplied from the strategic national stockpile for treatment of Covid-19 in certain hospitalized patients. Available at: https://www.fda.gov/media/136537/download. Accessed 15 Nov 2020
Ferner RE, Aronson JK (2020) Chloroquine and hydroxychloroquine in covid-19. BMJ 369:m1432. https://doi.org/10.1136/bmj.m1432
Ferraz L, Santos F, Ferreira A, Junior RTLM, Rosa TA, Rolim LA, Rolim-Neto PJ (2014) Clinical, pharmacokinetic and technological aspects of the hydroxychloroquine sulfate. IOSR J Pharm 4(11):53–64. https://doi.org/10.9790/3013-04011053064
Fleming SB (2016) Viral inhibition of the IFN-induced JAK/STAT signalling pathway: development of live attenuated vaccines by mutation of viral-encoded IFN-antagonists. Vaccines (Basel) 4(3):23. https://doi.org/10.3390/vaccines4030023
Fu B, Xu X, Wei H (2020) Why tocilizumab could be an effective treatment for severe COVID-19? J Transl Med 18:164. https://doi.org/10.1186/s12967-020-02339-3
Furst DE (1996) Pharmacokinetics of hydroxychloroquine and chloroquine during treatment of rheumatic diseases. Lumpus Suppl 1:S11–S15. https://doi.org/10.1177/0961203396005001041
Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, Dupont HT, Honoré S, Colson P, Chabrière E, La Scola B, Rolain JM et al (2020) Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents:105949. https://doi.org/10.1016/j.ijantimicag.2020.105949
Geleris J, Sun Y, Platt J, Zucker J, Baldwin M, Hripcsak G, Labella A, Manson D, Kubin C, Barr RG, Sobieszczyk ME, Schluger NW (2020) Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med 382:2411–2418. https://doi.org/10.1056/NEJMoa2012410
Golden EB, Cho HY, Hofman FM, Louie SG, Schönthal AH, Chen TC (2015) Quinoline-based antimalarial drugs: a novel class of autophagy inhibitors. Neurosurg Focus 38(3):E12. https://doi.org/10.3171/2014.12.FOCUS14748
Gonçalves C, Perez S, Osorio V, Petrovic M, Alpendurada MF, Barcelo D (2011) Photofate of oseltamivir (Tamiflu) and oseltamivir carboxylate under naturaland simulated solar irradiation: kinetics, identification of the transformationproducts, and environmental occurrence. Environ Sci Technol 45:4307e4314–4307e4314. https://doi.org/10.1021/es1032629
Gordon CJ, Tchesnokov EP, Feng JY, Porter DP, Gotte M (2020) The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J Biol Chem 295(15):4773–4779. https://doi.org/10.1074/jbc.AC120.013056
Guastalegname M, Vallone A (2020) Could chloroquine /hydroxychloroquine be harmful in Coronavirus Disease 2019 (COVID-19) treatment? Clin Infect Dis ciaa321. https://doi.org/10.1093/cid/ciaa321
Hacker H, Mischak H, Miethke T, Liptay S, Schmid R et al (1998) CpG-DNA-specific activation of antigen-presenting cells requires stress kinase activity and is preceded by non-specific endocytosis and endosomal maturation. EMBO J 17:6230–6240. https://doi.org/10.1093/emboj/17.21.6230
Hasana S, Hossain MF, Jalouli M, Kabir MT, Uddin MG, Wahed MII, Behl T, Bin-Jumah MN, Abdel-Daim MM, Aleya L, Uddin MS (2021) Genetic Diversity of SARS-CoV2 and environmental settings: possible association with neurological disorders. Mol Neurobiol. https://doi.org/10.1007/s12035-020-02239-z
Hemilä H (2003) Vitamin C and SARS coronavirus. J Antimicrob Chemother 52(6):1049–1050. https://doi.org/10.1093/jac/dkh002
Hemilä H, Douglas RM (1999) Vitamin C and acute respiratory infections. Int J Tuberc Lung Dis 3(9):756–761
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395(10223):497–506. https://doi.org/10.1016/S0140-6736(20)30183-5
Jean SS, Lee PI, Hsueh PR (2020) Treatment options for COVID-19: the reality and challenges. J Microbiol Immunol Infect 53(3):436–443. https://doi.org/10.1016/j.jmii.2020.03.034
Jeon S, Ko M, Lee J, Choi I, Byun SY, Park S, Shum D, Kim S (2020) Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. bioRxiv. https://doi.org/10.1101/2020.03.20.999730
Jorge AM, Melles RB, Zhang Y, Lu N, Rai SK, Young LH, Costenbader KH, Ramsey-Goldman R, Lim SS, Esdaile JM, Clarke AE, Urowitz MB, Askanase A, Aranow C, Petri M, Choi H (2018) Hydroxychloroquine prescription trends and predictors for excess dosing per recent ophthalmology guidelines. Arthritis Res Ther 20:133. https://doi.org/10.1186/s13075-018-1634-8
Kabir MT, Uddin MS, Hossain MF, Abdulhakim JA, Alam MA, Ashraf GM, Bungau SG, Bin-Jumah MN, Abdel-Daim MM, Aleya L (2020) nCOVID-19 pandemic: from molecular pathogenesis to potential investigational therapeutics. Front Cell Dev Biol 8:616. https://doi.org/10.3389/fcell.2020.00616
Kanoh S, Rubin BK (2010) Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev 23:590–615. https://doi.org/10.1128/CMR.00078-09
Kawabata K, Sugihara K, Sanoh S, Kitamura S, Ohta S (2013) Photodegradation of pharmaceuticals in the aquatic environment by sunlight and UV-A, -B and -Cirradiation. J Toxicol Sci 38:215e223–215e223. https://doi.org/10.2131/jts.38.215
Khalil SA (1977) Effect of chloroquine adsorption on acid reactivity of magnesium trisilicate. J Pharm Sci 66(2):289–290. https://doi.org/10.1002/jps.2600660244
Kim KA, Park JY, Lee JS, Lim S (2003) Cytochrome P450 2C8 and CYP3A4/5 are involved in chloroquine metabolism in human liver microsomes. Arch Pharm Res 26(8):631–637. https://doi.org/10.1007/BF02976712
Ko WC, Rolain JM, Lee NY, Chen PL, Huang CT, Lee PI, Hsueh PR (2020) Arguments in favor of remdesivir for treating SARS-CoV-2 infections. Int J Antimicrob Agents 55(4):105933. https://doi.org/10.1016/j.ijantimicag.2020.105933
Ksiazek TG, Erdman D, Goldsmith CS (2003) A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med 348(20):1953–1966. https://doi.org/10.1056/NEJMoa030781
Kumar R, Sharma A, Siddiqui MH, Tiwari RK (2016) Prediction of metabolism of drugs using artificial intelligence: how far have we reached? Curr Drug Metab 17(2):129–141. https://doi.org/10.2174/1389200216666151103121352
Kumar R, Srivastava JK, Singh R, Siddiqui MH, Mansouri RA, Abdulhakim JA, Bin-Jumah MN, Alkahtani S, Abdel-Daim MM, Uddin MS (2020) Available compounds with therapeutic potential against COVID-19: antimicrobial therapies, supportive care, and probable vaccines. Front Pharmacol 11:582025. https://doi.org/10.3389/fphar.2020.582025
Leden I (1982) Digoxin-hydroxychloroquine interaction? Acta Med Scand 211(5):411–4122. https://doi.org/10.1111/j.0954-6820.1982.tb01971.x
Lee AJ, Ashkar AA (2018) The dual nature of type I and type II interferons. Front Immunol 9:2061. https://doi.org/10.3389/fimmu.2018.02061
Ling Ngan Wong A, Tsz Fung Cheung I, Graham CA (2008) Hydroxychloroquine overdose: case report and recommendations for management. Eu J Emerg Med 15(1):16–18. https://doi.org/10.1097/MEJ.0b013e3280adcb56
Liu X, Wang XJ (2020) Potential inhibitors for 2019-nCoV coronavirus M protease from clinically proven medicines. J Genet Genomics 47(2):119–121. https://doi.org/10.1016/j.jgg.2020.02.001
Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, Li Y, Hu Z, Zhong W, Wang M (2020) Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov 6:16. https://doi.org/10.1038/s41421-020-0156-0
Lu CC, Chen MY, Chang YL (2020) Potential therapeutic agents against COVID-19: what we know so far. J Chin Med Assoc 83:534–536. https://doi.org/10.1097/JCMA.0000000000000318
Lui RN, Wong SH, Sánchez-Luna SA, Pellino G, Bollipo S, Wong MY, Chiu PWY, Sung JJY (2020) Overview of guidance for endoscopy during the coronavirus disease 2019 pandemic. J Gastroenterol Hepatol 35(5):749–759. https://doi.org/10.1111/jgh.15053
Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J (2020) Tocilizumab treatment in COVID-19: a single center experience. J Med Virol 92:814–818. https://doi.org/10.1002/jmv.25801
Macfarlane DE, Manzel L (1998) Antagonism of immunostimulatory CpG-oligodeoxynucleotides by quinacrine, chloroquine, and structurally related compounds. J Immunol 160:1122–1131
Mack HG (2020) Hydroxychloroquine use during the COVID-19 pandemic 2020. Aust J Gen Pract 49. https://doi.org/10.31128/AJGP-COVID-08
Marmor MF, Kellner U, Lai TY, Lyons JS, Mieler WF (2011) Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology 118(2):415–422. https://doi.org/10.1016/j.ophtha.2010.11.017
Matsuyama S, Kawase M, Nao N, Shirato K, Ujike M, Kamitani W, Shimojima M, Fukushi S (2020) The inhaled corticosteroid ciclesonide blocks coronavirus RNA replication by targeting viral NSP15. bioRxiv. https://doi.org/10.1101/2020.03.11.987016
McChesney EW (1983) Animal toxicity and pharmacokinetics of hydroxychloroquine sulfate. Am J Med 75:11–18. https://doi.org/10.1016/0002-9343(83)91265-2
McElnay JC, Mukhtar HA, D'Arcy PF, Temple DJ, Collier PS (1982) The effect of magnesium trisilicate and kaolin on the in vivo absorption of chloroquine. J Trop Med Hyg 85(4):159–163
Menachery VD, Yount BL Jr, Debbink K, Agnihothram S, Gralinski LE, Plante JA, Graham RL, Scobey T, Ge XY, Donaldson EF, Randell SH, Lanzavecchia A, Marasco WA, Shi ZL, Baric RS (2015) SARS-like cluster of circulating bat coronaviruses shows potential for human emergence. Nat Med 21(12):1508–1513. https://doi.org/10.1038/nm.3985
Mitjà O, Clotet B (2020) Use of antiviral drugs to reduce COVID-19 transmission. Lancet Glob Health 8(5):e639–e640. https://doi.org/10.1016/S2214-109X(20)30114-5
Molina JM, Delaugerre C, Le Goff J, Mela-Lima B, Ponscarme D, Goldwirt L, de Castro N (2020) No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med Mal Infect S0399-077X(20):30085–30088. https://doi.org/10.1016/j.medmal.2020.03.006
Nallar SC, Kalvakolanu DV (2014) Interferons, signal transduction pathways, and the central nervous system. J Interf Cytokine Res 34(8):559–576. https://doi.org/10.1089/jir.2014.0021
Nampoory MR, Nessim J, Gupta RK, Johny KV (1992) Drug interaction of chloroquine with ciclosporin. Nephron 62(1):108–109. https://doi.org/10.1093/jac/dkg319/10.1159/000187007
National Health Commission of the People's Republic of China Interpretation of COVID-19 treatment guidelines (6th version) (2020). http://www.gov.cn/zhengce/2020-02/19/content_5480958.htm. Accessed 6 November 2020
Popert AJ (1976) Choloroquine: a review. Rheumatology 15:235–238. https://doi.org/10.1093/rheumatology/15.3.235
Ramesh M, Anitha S, Poopal RK, Shobana C (2017) Evaluation of acute and sublethal effects of chloroquine (C18H26CIN3) on certain enzymological and histopathological biomarker responses of a freshwater fish Cyprinus carpio. Toxicol Rep 5:18–27. https://doi.org/10.1016/j.toxrep.2017.11.006
Rathi S, Ish P, Kalantri A, Kalantri S (2020) Hydroxychloroquine prophylaxis for COVID-19 contacts in India. The Lancet Infect Dis 20:1118–1119. https://doi.org/10.1016/S1473-3099(20)30313-3
Richardson PJ, Corbellino M, Stebbing J (2020) Baricitinib for COVID-19: a suitable treatment? Lancet Infect Dis S1473:3099(20)30270-X. https://doi.org/10.1016/S1473-3099(20)30270-X
Rolain JM, Colson P, Raoult D (2007) Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century. Int J Antimicrob Agents 30:297–308. https://doi.org/10.1016/j.ijantimicag.2007.05.015
Rosenberg ES, Dufort EM, Udo T, Wilberschied LA, Kumar J et al (2020) Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York state. JAMA:e208630. https://doi.org/10.1001/jama.2020.8630
Rubin EJ, Harrington DP, Hogan JW, Gatsonis C, Baden LR et al (2020) The urgency of care during the Covid-19 pandemic - learning as we go. N Engl J Med. NEJMe2015903. https://doi.org/10.1056/NEJMe2015903
Sahraei Z, Shabani M, Shokouhi S, Saffaei A (2020) Aminoquinolines against coronavirus disease 2019 (COVID-19): chloroquine or hydroxychloroquine. Int. J. Antimicrob. Agents 55(4):105945. https://doi.org/10.1016/j.ijantimicag.2020.105945
Sanofi (2020) Important information on Plaquenil®/Quensyl®/Plaquinol® (hydroxychloroquine) and COVID-19. Available at: https://www.sanofi.com/en/about-us/our-stories/important-information-on-plaquenil-quensyl-plaquinol-COVID-19. Accessed 15 Nov 2020
Sanofi internal report (2016) Hydroxychloroquine (CAS N°118–42-3) Ready Biodegradability “Manometric Respirometry. OECD 301F. Report BPL15–0032 EB
Savarino A, Di Trani L, Donatelli I, Cauda R, Cassone A (2006) New insights into the antiviral effects of chloroquine. Lancet Infect Dis 2006:667–169. https://doi.org/10.1016/S1473-3099(06)70361-9
Schrezenmeier E, Dörner T (2020) Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol 16:155–166. https://doi.org/10.1038/s41584-020-0372-x
Shehab N, Lovegrove M, Budnitz DS (2020) US hydroxychloroquine, chloroquine, and azithromycin outpatient prescription trends, October 2019 through March 2020. JAMA Intern Med 180(10):1384–1386. https://doi.org/10.1001/jamainternmed.2020.2594
Siddiqui AJ, Danciu C, Ashraf SA, Moin A, Singh R, Alreshidi M, Patel M, Jahan S, Kumar S, Alkhinjar MIM, Badraoui R, Snoussi M, Adnan M (2020a) Plants-derived biomolecules as potent antiviral phytomedicines: new insights on ethnobotanical evidences against coronaviruses. Plants (Basel) 9(9):1244. https://doi.org/10.3390/plants9091244
Siddiqui AJ, Jahan S, Ashraf SA, Alreshidi M, Ashraf MS, Patel et al (2020b) Current status and strategic possibilities on potential use of combinational drug therapy against COVID-19 caused by SARS-CoV-2. J Biomol Struct Dyn:1–14. https://doi.org/10.1080/07391102.2020.1802345
Şimşek Yavuz S, Ünal S (2020) Antiviral treatment of COVID-19. Turk J Med Sci 50(SI-1):611–619. https://doi.org/10.3906/sag-2004-145
Singh AK, Singh A, Shaikh A, Singh R, Misra A (2020) Chloroquine and hydroxychloroquine in the treatment of COVID-19 with or without diabetes: a systematic search and a narrative review with a special reference to India and other developing countries. Diabetes Metab Syndr 14(3):241–246. https://doi.org/10.1016/j.dsx.2020.03.011
Sinha N, Balayla G (2020) Hydroxychloroquine and covid-19. Postgrad Med J postgradmedj-2020:137785. https://doi.org/10.1136/postgradmedj-2020-137785
Somer M, Kallio J, Pesonen U, Pyykkö K, Huupponen R, Scheinin M (2000) Influence of hydroxychloroquine on the bioavailability of oral metoprolol. Br J Clin Pharmacol 49(6):549–554. https://doi.org/10.1046/j.1365-2125.2000.00197.x
Stebbing J, Phelan A, Griffin I, Tucker C, Oechsle O, Smith D, Richardson P (2020) COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis 20(4):400–402. https://doi.org/10.1016/S1473-3099(20)30132-8
Stokkermans TJ, Trichonas G (2020) Chloroquine and hydroxychloroquine toxicity. In: StatPearls. StatPearls Publishing, Treasure Island
Surti M, Patel M, Adnan M, Moinc A, Ashraf SA et al (2020) Ilimaquinone (marine sponge metabolite) as a novel inhibitor of SARS-CoV-2 key target proteins in comparison with suggested COVID-19 drugs: designing, docking and molecular dynamics simulation study. RSC Adv 10:37707–37720. https://doi.org/10.1039/D0RA06379G
Tan YW, Yam WK, Sun J, Chu JJH (2018) An evaluation of chloroquine as a broad-acting antiviral against Hand, Foot and Mouth Disease. Antivir Res 149:143–149. https://doi.org/10.1016/j.antiviral.2017.11.017
Touret F, de Lamballerie X (2020) Of chloroquine and COVID-19. Antivir Res 177:104762. https://doi.org/10.1016/j.antiviral.2020.104762
Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, Seidah NG, Nichol ST (2005) Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J 2:69. https://doi.org/10.1186/1743-422X-2-69
Wang C, Horby PW, Hayden FG, Gao F (2020a) A novel coronavirus outbreak of global health concern. Lancet 395(10223):470–473. https://doi.org/10.1016/S0140-6736(20)30185-9
Wang M, Cao R, Zang L, Yang X, Liu J (2020b) Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 30:269–271. https://doi.org/10.1038/s41422-020-0282-0
Warhurst DC, Steele JC, Adagu IS, Craig JC, Cullander C (2003) Hydroxychloroquine is much less active than chloroquine against chloroquine-resistant Plasmodium falciparum, in agreement with its physicochemical properties. J Antimicrob Chemother 52(2):188–193. https://doi.org/10.1093/jac/dkg319
Warren TK, Jordan R, Lo MK, Ray AS, Mackman RL, Soloveva V, Siegel D, Perron M, Bannister R, Hui HC, Larson N, Strickley R, Wells J, Stuthman KS, van Tongeren SA, Garza NL, Donnelly G, Shurtleff AC, Retterer CJ, Gharaibeh D, Zamani R, Kenny T, Eaton BP, Grimes E, Welch LS, Gomba L, Wilhelmsen CL, Nichols DK, Nuss JE, Nagle ER, Kugelman JR, Palacios G, Doerffler E, Neville S, Carra E, Clarke MO, Zhang L, Lew W, Ross B, Wang Q, Chun K, Wolfe L, Babusis D, Park Y, Stray KM, Trancheva I, Feng JY, Barauskas O, Xu Y, Wong P, Braun MR, Flint M, McMullan LK, Chen SS, Fearns R, Swaminathan S, Mayers DL, Spiropoulou CF, Lee WA, Nichol ST, Cihlar T, Bavari S (2016) Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 531:381–385. https://doi.org/10.1038/nature17180
Wondafrash DZ, Desalegn TZ, Yimer EM, Tsige AG, Adamu BA, Zewdie KA (2020) Potential effect of hydroxychloroquine in diabetes mellitus: a systematic review on preclinical and clinical trial studies. J Diabetes Res 2020:5214751–5214710. https://doi.org/10.1155/2020/5214751
Wu C, Liu Y, Yang Y, Zhang P, Zhong W, Wang Y, Wang Q, Xu Y, Li M, Li X, Zheng M, Chen L, Li H (2020) Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B 10:766–788. https://doi.org/10.1016/j.apsb.2020.02.008
Yan Y, Zou Z, Sun Y, Li X, Xu KF, Wei Y, Jin N, Jiang C (2013) Anti-malaria drug chloroquine is highly effective in treating avian influenza A H5N1 virus infection in an animal model. Cell Res 23:300–302. https://doi.org/10.1038/cr.2012.165
Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, Liu X, Zhao L, Dong E et al (2020) In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis:ciaa237. https://doi.org/10.1093/cid/ciaa237
Yazdany J, Kim AHJ (2020) Use of hydroxychloroquine and chloroquine during the COVID-19 pandemic: what every clinician should know. Ann Intern Med 172:M20–M1334. https://doi.org/10.7326/M20-1334
Yu B, Li C, Chen P, Zhou N, Wang L, Li J et al (2020) Hydroxychloroquine application is associated with a decreased mortality in critically ill patients with COVID-19. https://doi.org/10.1101/2020.04.27.20073379
Zaki AM, Van Boheemen S, Bestebroer TM (2012) Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 367(19):1814–1820. https://doi.org/10.1056/NEJMoa1211721
Zarogoulidis P, Papanas N, Kioumis I, Chatzaki E, Maltezos E, Zarogoulidis K (2012) Macrolides: from in vitro anti-inflammatory and immunomodulatory properties to clinical practice in respiratory disease. Eur J Clin Pharmacol 68(5):479–503. https://doi.org/10.1007/s00228-011-1161-x
Zhai P, Ding Y, Wu X, Long J, Zhong Y, Li Y (2020) The epidemiology, diagnosis and treatment of COVID-19. Int J Antimicrob Agents 105955:105955. https://doi.org/10.1016/j.ijantimicag.2020.105955
Zhang J, Wang W, Peng B, Peng W, Zhang Y, Wang Y, Wan Y, Chang J et al (2020) Potential of Arbidol for post-exposure prophylaxis of COVID-19 transmission. ChinaXiv:202002.00065. https://doi.org/10.12074/202002.00065
Zhu H, Wei L, Niu P (2020) The novel coronavirus outbreak in Wuhan, China. Glob Health Res Policy 5:6. https://doi.org/10.1186/s41256-020-00135-6
Zurita JL, Jos A, del Peso A, Salguero M, López-Artíguez M, Repetto G (2005) Ecotoxicological evaluation of the antimalarial drug chloroquine. Aquat Toxicol 75(2):97–107. https://doi.org/10.1016/j.aquatox.2005.07.009
Acknowledgments
The authors concede the support by the Pharmakon Neuroscience Research Network, Dhaka, Bangladesh.
Author information
Authors and Affiliations
Contributions
RK, AS, JKS, and MHS wrote the draft of the manuscript and prepared the figures. MSU and LA performed the literature review and improved the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, R., Sharma, A., Srivastava, J.K. et al. Hydroxychloroquine in COVID-19: therapeutic promises, current status, and environmental implications. Environ Sci Pollut Res 28, 40431–40444 (2021). https://doi.org/10.1007/s11356-020-12200-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-12200-1