Abstract
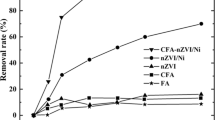
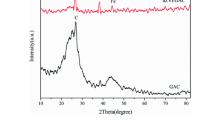
The present study encompasses a unique concept involving the formation of core-shell particles with surface-activated fly ash (FA) as core and nanoscale zerovalent iron (nZVI) particles as shell, which not only imparts high adsorption efficiency for Cr(VI) but also contributes to fruitful utilization of FA while overcoming the drawbacks associated with ZVI nanoparticles (aggregation, rapid oxidation and less durability). The otherwise inert surface of FA has been modified and activated to achieve a uniform and stable layer of nZVI over FA. The functionalized particles were studied using FE-SEM/EDAX, HR-TEM, XRD and FT-IR studies for its physical, functional and morphological characteristics. The results indicate the strong adsorption ability of nZVI@FA particles, with 100% removal efficiency within 10 min at low initial concentrations of Cr(VI), which is appreciably higher than that of pure fly ash (26%) after 60 min of reaction. Besides, the so-formed structure of composite aids to improve its life, as the synthesized nZVI@FA particles could be efficiently regenerated and reused up to 5 subsequent adsorption-desorption cycles, which is in contrast with the ability of fly ash considering its low desorption potential. Hence, the composite material proves to be an effective and sustainable alternative for treatment of a waste using a waste.
Graphical Abstract












Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article (and its supplementary information files).
References
Agrawal A, Tratnyek PG (1995) Reduction of nitro aromatic compounds by zero-valent iron metal. Environ Sci Technol 30:153–160
Arshadi M, Foroughifard S, Gholtash JE, Abbaspourrad A (2015) Preparation of iron nanoparticles-loaded Spondias purpurea seed waste as an excellent adsorbent for removal of phosphate from synthetic and natural waters. J Colloid Interface Sci 452:69–77
Ayanda OS, Fatoki OS, Adekola FA, Ximba BJ (2013) Utilization of nSiO 2, fly ash, and nSiO 2/fly ash composite for the remediation of triphenyltin (TPT) from contaminated seawater. Environ Sci Pollut Res 20:8172–8181
Baikousi M, Bourlinos AB, Douvalis A, Bakas T, Anagnostopoulos DF, Ji T, Šafářová K, Zboril R, Karakassides MA (2012) Synthesis and characterization of γ-Fe2O3/carbon hybrids and their application in removal of hexavalent chromium ions from aqueous solutions. Langmuir 28:3918–3930
Banerjee S, Joshi M, Jayaram R (2005) Removal of Cr (VI) and Hg (II) from aqueous solutions using fly ash and impregnated fly ash. Sep Sci Technol 39:1611–1629
Bayramoğlu G, Çelik G, Yalçın E, Yılmaz M, Arıca MY (2005) Modification of surface properties of Lentinus sajor-caju mycelia by physical and chemical methods: evaluation of their Cr6+ removal efficiencies from aqueous medium. J Hazard Mater 119:219–229
Beganskienė A, Sirutkaitis V, Kurtinaitienė M, Juškėnas R, Kareiva A (2004) FTIR, TEM and NMR investigations of Stöber silica nanoparticles. Mater Sci (Medžiagotyra) 10:287–290
Canafoglia ME, Lick ID, Ponzi EN, Botto IL (2009) Natural materials modified with transition metals of the cobalt group: feasibility in catalysis. J Argent Chem Soc 97:58–68
Di Natale F, Lancia A, Molino A, Musmarra D (2007) Removal of chromium ions form aqueous solutions by adsorption on activated carbon and char. J Hazard Mater 145:381–390
Feng Z, Chen N, Feng C, Gao Y (2018) Mechanisms of Cr (VI) removal by FeCl3-modified lotus stem-based biochar (FeCl3@ LS-BC) using mass-balance and functional group expressions. Colloids Surf A Physicochem Eng Asp 551:17–24
Gotić M, Musić S (2007) Mössbauer, FT-IR and FE SEM investigation of iron oxides precipitated from FeSO4 solutions. J Mol Struct 834:445–453
Gupta V, Pathania D, Agarwal S, Sharma S (2013) Removal of Cr (VI) onto Ficus carica biosorbent from water. Environ Sci Pollut Res 20:2632–2644
He C, Yang Z, Ding J, Chen Y, Tong X, Li Y (2017) Effective removal of Cr (VI) from aqueous solution by 3-aminopropyltriethoxysilane-functionalized graphene oxide. Colloids Surf A Physicochem Eng Asp 520:448–458
He R, Yuan X, Huang Z, Wang H, Jiang L, Huang J, Tan M, Li H (2019a) Activated biochar with iron-loading and its application in removing Cr (VI) from aqueous solution. Colloids Surf A Physicochem Eng Asp 579:123642
He Y, Zhang L, An X, Han C, Luo Y (2019b) Microwave assistant rapid synthesis MCM-41-NH 2 from fly ash and Cr (VI) removal performance. Environ Sci Pollut Res 26:31463–31477
Ho Y-S, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Huang L, Zhou S, Jin F, Huang J, Bao N (2014) Characterization and mechanism analysis of activated carbon fiber felt-stabilized nanoscale zero-valent iron for the removal of Cr (VI) from aqueous solution. Colloids Surf A Physicochem Eng Asp 447:59–66
Jiang X, Fan W, Li C, Wang Y, Bai J, Yang H, Liu X (2019) Removal of Cr (VI) from wastewater by a two-step method of oxalic acid reduction-modified fly ash adsorption. RSC Adv 9:33949–33956
Joshi RC, Lohita R (1997) Fly ash in concrete: production, properties and uses, Advances in concrete technology, Volume 2
Karanac M, Đolić M, Veljović Đ, Rajaković-Ognjanović V, Veličković Z, Pavićević V, Marinković A (2018) The removal of Zn2+, Pb2+, and As (V) ions by lime activated fly ash and valorization of the exhausted adsorbent. Waste Manag 78:366–378
Kimbrough DE, Cohen Y, Winer AM, Creelman L, Mabuni C (1999) A critical assessment of chromium in the environment. Crit Rev Environ Sci Technol 29:1–46
Lagergren S (1898) About the theory of so-called adsorption of soluble substances. Sven Vetenskapsakad Handingarl 24:1–39
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J 503 Am Chem Soc pp. 504
Li J, Lin Q, Zhang X, Yan Y (2009) Kinetic parameters and mechanisms of the batch biosorption of Cr (VI) and Cr (III) onto Leersia hexandra Swartz biomass. J Colloid Interface Sci 333:71–77
Li X, Zhao Y, Xi B, Mao X, Gong B, Li R, Peng X, Liu H (2016) Removal of nitrobenzene by immobilized nanoscale zero-valent iron: effect of clay support and efficiency optimization. Appl Surf Sci 370:260–269
Li Z, Lowry GV, Fan J, Liu F, Chen J (2018a) High molecular weight components of natural organic matter preferentially adsorb onto nanoscale zero valent iron and magnetite. Sci Total Environ 628:177–185
Li Z, Wang L, Meng J, Liu X, Xu J, Wang F, Brookes P (2018b) Zeolite-supported nanoscale zero-valent iron: new findings on simultaneous adsorption of Cd (II), Pb (II), and As (III) in aqueous solution and soil. J Hazard Mater 344:1–11
Liu T, Rao P, Lo IM (2009) Influences of humic acid, bicarbonate and calcium on Cr (VI) reductive removal by zero-valent iron. Sci Total Environ 407:3407–3414
Liu T, Zhao L, Wang Z (2012) Removal of hexavalent chromium from wastewater by Fe0-nanoparticles-chitosan composite beads: characterization, kinetics and thermodynamics. Water Sci Technol 66:1044–1051
Lu H, Wen C, Gao S, Dong Y, Zhang M, Li B, Hu W, Dong J (2018) Incorporation of nanoscale zero-valent iron particles in monodisperse mesoporous silica nanospheres: characterization, reactivity, transport in porous media. Colloids Surf A Physicochem Eng Asp 553:28–34
Lv X, Xu J, Jiang G, Xu X (2011) Removal of chromium (VI) from wastewater by nanoscale zero-valent iron particles supported on multiwalled carbon nanotubes. Chemosphere 85:1204–1209
Marinho BA, Cristóvão RO, Boaventura RA, Vilar VJ (2019) As (III) and Cr (VI) oxyanion removal from water by advanced oxidation/reduction processes—a review. Environ Sci Pollut Res 26:2203–2227
Namasivayam C, Ranganathan K (1993) Waste Fe (III)/Cr (III) hydroxide as adsorbent for the removal of Cr (VI) from aqueous solution and chromium plating industry wastewater. Environ Pollut 82:255–261
Nguyen TC, Loganathan P, Nguyen TV, Kandasamy J, Naidu R, Vigneswaran S (2018) Adsorptive removal of five heavy metals from water using blast furnace slag and fly ash. Environ Sci Pollut Res 25:20430–20438
Ponder SM, Darab JG, Mallouk TE (2000) Remediation of Cr (VI) and Pb (II) aqueous solutions using supported, nanoscale zero-valent iron. Environ Sci Technol 34:2564–2569
Pradhan J, Das SN, Thakur RS (1999) Adsorption of hexavalent chromium from aqueous solution by using activated red mud. J Colloid Interface Sci 217:137–141
Qin L, He L, Yang W, Lin A (2020) Preparation of a novel iron-based biochar composite for removal of hexavalent chromium in water. Environ Sci Pollut Res:1–13
Selomulya C, Meeyoo V, Amal R (1999) Mechanisms of Cr (VI) removal from water by various types of activated carbons. J Chem Technol Biotechnol 74:111–122
Sharma R, Shaw R, Tiwari S, Tiwari S (2015) Nano-titania decorated fly ash as self-cleaning antibacterial cool pigment. ACS Sustain Chem Eng 3:2796–2803
Sheng G, Tang Y, Linghu W, Wang L, Li J, Li H, Wang X, Huang Y (2016) Enhanced immobilization of ReO4− by nanoscale zerovalent iron supported on layered double hydroxide via an advanced XAFS approach: implications for TcO4− sequestration. Appl Catal B Environ 192:268–276
Shi L-n, Lin Y-M, Zhang X, Chen Z-l (2011a) Synthesis, characterization and kinetics of bentonite supported nZVI for the removal of Cr (VI) from aqueous solution. Chem Eng J 171:612–617
Shi L-n, Zhang X, Chen Z-l (2011b) Removal of chromium (VI) from wastewater using bentonite-supported nanoscale zero-valent iron. Water Res 45:886–892
Shukla S, Seal S, Akesson J, Oder R, Carter R, Rahman Z (2001) Study of mechanism of electroless copper coating of fly-ash cenosphere particles. Appl Surf Sci 181:35–50
Simate GS, Maledi N, Ochieng A, Ndlovu S, Zhang J, Walubita LF (2016) Coal-based adsorbents for water and wastewater treatment. J Environ Chem Eng 4:2291–2312
Song Y, Wang L, Lv B, Chang G, Jiao W, Liu Y (2020) Removal of trace Cr (VI) from aqueous solution by porous activated carbon balls supported by nanoscale zero-valent iron composites. Environ Sci Pollut Res 27:7015–7024
Srivastava S, Gupta V, Mohan D (1997) Removal of lead and chromium by activated slag—a blast-furnace waste. J Environ Eng 123:461–468
Su C, Puls RW (2001) Arsenate and arsenite removal by zerovalent iron: effects of phosphate, silicate, carbonate, borate, sulfate, chromate, molybdate, and nitrate, relative to chloride. Environ Sci Technol 35:4562–4568
Tytłak A, Oleszczuk P, Dobrowolski R (2015) Sorption and desorption of Cr (VI) ions from water by biochars in different environmental conditions. Environ Sci Pollut Res 22:5985–5994
Umpleby RJ II, Baxter SC, Bode M, Berch JK Jr, Shah RN, Shimizu KD (2001) Application of the Freundlich adsorption isotherm in the characterization of molecularly imprinted polymers. Anal Chim Acta 435:35–42
Wen Y, Tang Z, Chen Y, Gu Y (2011) Adsorption of Cr (VI) from aqueous solutions using chitosan-coated fly ash composite as biosorbent. Chem Eng J 175:110–116
Wilbur S, Abadin H, Fay M, Yu D, Tencza B, Ingerman L, Klotzbach J, James S (2012) Health effects, Toxicological profile for chromium. Agency for Toxic Substances and Disease Registry (US)
Withers PJ, Bhadeshia H (2001) Residual stress. Part 1–measurement techniques. Mater Sci Technol 17:355–365
Zhang H, Lü J, Peng J, Du G, Peng H, Fang Y (2018) One-step preparation of emulsion-templated amino-functionalized porous organosilica monoliths for highly efficient Cr (VI) removal. Colloids Surf A Physicochem Eng Asp 555:8–17
Zhao S, Chen Z, Shen J, Qu Y, Wang B, Wang X (2017) Enhanced Cr (VI) removal based on reduction-coagulation-precipitation by NaBH4 combined with fly ash leachate as a catalyst. Chem Eng J 322:646–656
Zhou Y, Gao B, Zimmerman AR, Chen H, Zhang M, Cao X (2014) Biochar-supported zerovalent iron for removal of various contaminants from aqueous solutions. Bioresour Technol 152:538–542
Zhu S, Huang X, Wang D, Wang L, Ma F (2018) Enhanced hexavalent chromium removal performance and stabilization by magnetic iron nanoparticles assisted biochar in aqueous solution: mechanisms and application potential. Chemosphere 207:50–59
Acknowledgements
The authors would like to thank the Department of Science and Technology, Government of India, for their financial support (DST/INT/PORTUGAL/P-07/2017).
Funding
The authors thank the Department of Science and Technology, Government of India, for providing financial support (Sanction No. DST/INT/PORTUGAL/P-07/2017) to carry out the experiments and for facilitating collaboration with Portugal scientist’s (including the visits).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study, concept and design. Material preparation, investigation, analysis and writing of the original draft of manuscript were performed by Shubhangi Madan, and Urvashi Thapa assisted in material preparation and investigation. Supervision, funding acquisition, project administration, conceptualization and data interpretation were the responsibilities of Sangeeta Tiwari, while Sandeep Kumar Tiwari was responsible for conceptualization, resources and data interpretation. Provision of resources, data curation and interpretation were performed by Suresh Kumar Jakka and Manuel Jorge Soares. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Additional information
Responsible Editor: Angeles Blanco
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Designing of nZVI@FA by uniform distribution of nZVI on industrial waste, fly ash.
• The composite structure overcomes the limitations of nZVI (aggregation and rapid oxidation).
• nZVI@FA effectively adsorb Cr(VI) and lower its concentration below permissible limit.
• The exhausted adsorbent could be reused for several adsorption-desorption cycles.
• The recyclability of nZVI@FA makes it a lucrative choice for environmental applications.
Supplementary information
ESM 1
(DOCX 1064 kb)
Rights and permissions
About this article
Cite this article
Madan, S., Thapa, U., Tiwari, S. et al. Designing of a nanoscale zerovalent iron@fly ash composite as efficient and sustainable adsorbents for hexavalent chromium (Cr(VI)) from water. Environ Sci Pollut Res 28, 22474–22487 (2021). https://doi.org/10.1007/s11356-020-11692-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11692-1