Abstract
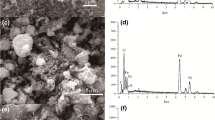
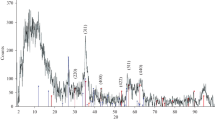
In this research, a novel γ-MnO2/chitosan/Fe3O4 nanocomposite was synthesized and modified by ethylenediaminetetraacetic acid (EDTA) for the separation and simultaneous elimination of Zn(II) and Pb(II) ions from aqueous solutions in a batch system. The magnetic nanocomposite was characterized by X-ray diffraction, Fourier transform infrared spectroscopy, scanning electron microscopy, transmission electron microscopy, and elemental analysis (EDAX). The results demonstrated that the magnetic nanocomposite was successfully synthesized and cross-linked. The predominant influential experimental parameters including pH, contact time, initial concentration, and temperature were analyzed in relation to the adsorption capacity. The experimental data were well converged with the double exponential kinetic model. Also, the results were well matched with the Langmuir isotherm, where the maximum adsorption values were 310.4 and 136 mg g−1 for Pb(II) and Zn(II), respectively. On the other hand, in the binary-component system, the Langmuir–Freundlich model dominated the experimental data. The thermodynamic results (ΔG° < 0, ΔH° > 0, and ΔS° > 0) within the temperature range of 25–40 °C showed that the nature of adsorption by the nanocomposite for both ions was spontaneous and endothermic and was favored at higher temperatures. The simultaneous removal of two ions, the excellent magnetic separation, and the high efficiency in reuse (five effective recovery cycles) indicated the high capability of the EDTA-modified γ-MnO2/chitosan/Fe3O4 nanocomposite in the treatment of industrial effluents from Pb(II) and Zn(II).












Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Aghazadeh M, Asadi M, Maragheh MG, Ganjali MR, Norouzi P, Faridbod F (2016) Facile preparation of MnO2 nanorods and evaluation of their supercapacitive characteristics. Appl Surf Sci 364:726–731. https://doi.org/10.1016/j.apsusc.2015.12.227
Bao S, Tang L, Li K, Ning P, Peng J, Guo H, Zhu T, Liu Y (2016) Highly selective removal of Cr(II) ion from hot-dip galvanizing pickling waste with amino-functionalized Fe3O4@SiO2 magnetic nano-adsorbent. J Colloid Interface Sci 462:235–242. https://doi.org/10.1016/j.jcis.2015.10.011
Bartholomew CJ, Li N, Li Y, Dai W, Nibagwire D, Guo T (2020) Characteristics and health risk assessment of heavy metals in street dust for children in Jinhua, China. Environ Sci Pollut Res 27(5):5042–5055. https://doi.org/10.1007/s11356-019-07144-0
Bashir S, Shaaban M, Hussain Q, Mehmood S, Zhu J, Fu Q, Aziz O, Hu H (2018a) Influence of organic and inorganic passivators on Cd and Pb stabilization and microbial biomass in a contaminated paddy soil. J Soils Sediments 18(9):2948–2959. https://doi.org/10.1007/s11368-018-1981-8
Bashir S, Zhu J, Fu Q, Hu H (2018b) Comparing the adsorption mechanism of Cd by rice straw pristine and KOH-modified biochar. Environ Sci Pollut Res 25(12):11875–11883. https://doi.org/10.1007/s11356-018-1292-z
Biswas S, Sen TK, Yeneneh AM, Meikap BC (2019) Synthesis and characterization of a novel Ca-alginate-biochar composite as efficient zinc (Zn2+) adsorbent: thermodynamics, process design, mass transfer and isotherm modeling. Sep Sci Technol 54:359–366. https://doi.org/10.1080/01496395.2018.1527353
Chao Y, Pang J, Bai Y, Wu P, Luo J, He J, Jin Y, Lee X, Xiong J, Li H, Zhu W (2020) Graphene-like BN@ SiO2 nanocomposites as efficient sorbents for solid-phase extraction of rhodamine B and rhodamine 6G from food samples. Food Chem 320:126666. https://doi.org/10.1016/j.foodchem.2020.126666
Chen B, Zhao H, Chen S, Long F, Huang B, Yang B, Pan X (2019) A magnetically recyclable chitosan composite adsorbent functionalized with EDTA for simultaneous capture of anionic dye and heavy metals in complex wastewater. Chem Eng J 356:69–80. https://doi.org/10.1016/j.cej.2018.08.222
Cui L, Wang Y, Gao L, Hu L, Yan L, Wei Q, Du B (2015) EDTA functionalized magnetic graphene oxide for removal of Pb(II), Hg(II) and Cu(II) in water treatment: adsorption mechanism and separation property. Chem Eng J 281:1–10. https://doi.org/10.1016/j.cej.2015.06.043
Dan L, Liu Y, Zhou J, Yang K, Lou Z, Baig SA, Xu X (2017) Application of EDTA functionalized bamboo activated carbon (BAC) for Pb(II) and Cu(II) removal from aqueous solutions. Appl Surf Sci 428:648–658. https://doi.org/10.1016/j.apsusc.2017.09.151
Depci T, Kul AR, Önal Y (2012) Competitive adsorption of lead and zinc from aqueous solution on activated carbon prepared from Van apple pulp: Study in single- and multi-solute systems, CHEM ENG J 200-202:224-236. https://doi.org/10.1016/j.cej.2012.06.077
Dinh VP, Li NC, Tuyen A, Hung NQ, Nguyen VD, Nguyen NT (2018) Insight into adsorption mechanism of lead(II) from aqueous solution by chitosan loaded MnO2 nanoparticles. Mater Chem Phys 207:294–302. https://doi.org/10.1016/j.matchemphys.2017.12.071
Facchi DP, Cazetta AL, Canesin EA, Almeida VC, Bonafe EG, Kipper MJ, Martins AF (2018) New magnetic chitosan/alginate/Fe3O4@SiO2 hydrogel composites applied for removal of Pb(II) ions from aqueous systems. Chem Eng J 337:595–608. https://doi.org/10.1016/j.cej.2017.12.142
Farokhi M, Parvareh A, Moraveji MK (2018) Performance of ceria/iron oxide nanocomposites based on chitosan as an effective adsorbent for removal of Cr(VI) and Co(II) ions from aqueous systems. Environ Sci Pollut Res 25:27059–27073. https://doi.org/10.1007/s11356-018-2594-x
Feng G, Ma J, Zhang X, Zhang Q, Xiao Y, Ma Q, Wang S (2019) Magnetic natural composite Fe3O4-chitosan@bentonite for removal of heavy metals from acid mine drainage. J Colloid Interface Sci 538:132–114. https://doi.org/10.1016/j.jcis.2018.11.087
Gong F, Cai H, Zhou B, Ou H (2018) The synthesis and characterization of AlPO4 hollow microspheres of uniform size, and the sorption properties for Pb2+, Cd2+, Cu2+, and Zn2+. Colloid Surface A 554:286–295. https://doi.org/10.1016/j.colsurfa.2018.06.027
Haldorai Y, Rengaraj A, Ryu T, Shin J, Huh YS, Han YK (2015) Response surface methodology for the optimization of lanthanum removal from an aqueous solution using a Fe3O4/chitosan nanocomposite. Mater Sci Eng B-Adv 195:20–29. https://doi.org/10.1016/j.mseb.2015.01.006
Han T, Zhang X, Fu X, Liu J (2017) Facile synthesis of chitosan nanoparticle-modified MnO2 nanoflakes for ultrafast adsorption of Pb(II) from aqueous solution. Water Supply 17:32–38. https://doi.org/10.2166/ws.2016.109
Huang J, Ye M, Qu Y, Chu L, Chen R, He Q, Xu D (2012) Pb(II) removal from aqueous media by EDTA-modified mesoporous silica SBA-15. J Colloid Interface Sci 385:137–146. https://doi.org/10.1016/j.jcis.2012.06.054
Iqbal A, Jan MR, Shah J, Rashid B (2020) Dispersive solid phase extraction of precious metal ions from electronic wastes using magnetic multiwalled carbon nanotubes composite. Miner Eng 154:106414. https://doi.org/10.1016/j.mineng.2020.106414
Kobylinska N, Kostenko L, Khainakov S, Garcia-Granda S (2020) Advanced core-shell EDTA-functionalized magnetite nanoparticles for rapid and efficient magnetic solid phase extraction of heavy metals from water samples prior to the multi-element determination by ICP-OES. Microchim Acta 187(5):289. https://doi.org/10.1007/s00604-020-04231-9
Kong A, Wang P, Zhang H, Yang F, Huang SP, Shan Y (2012) One-pot fabrication of magnetically recoverable acid nanocatalyst, heteropolyacids/chitosan/Fe3O4, and its catalytic performance. Appl Catal A-Gen 417-418:183–189. https://doi.org/10.1016/j.apcata.2011.12.040
Li TT, Liu YG, Peng QQ, Hu XJ, Liao T, Wang H, Lu M (2013) Removal of lead(II) from aqueous solution with ethylenediamine-modified yeast biomass coated with magnetic chitosan microparticles: kinetic and equilibrium modeling. Chem Eng J 214:189–197. https://doi.org/10.1016/j.cej.2012.10.055
Liang W, Lu Y, Li N, Li H, Zhu F (2020) Microwave-assisted synthesis of magnetic surface molecular imprinted polymer for adsorption and solid phase extraction of 4-nitrophenol in wastewater. Microchem J 159:105316. https://doi.org/10.1016/j.microc.2020.105316
Liu C, Wu T, Hsu PC, Xie J, Zhao J, Liu K, Sun J, Xu J, Tang J, Ye Z, Lin D, Cui Y (2019) Direct/alternating current electrochemical method for removing and recovering heavy metal from water using graphene oxide electrode. ACS Nano 13:6431–6437
Liu Y, Xiong Y, Xu P, Pang Y, Du C (2020) Enhancement of Pb (II) adsorption by boron doped ordered mesoporous carbon: isotherm and kinetics modeling. Sci Total Environ 708:134918. https://doi.org/10.1016/j.scitotenv.2019.134918
Lutke SF, Igansi AV, Pegoraro L, Dotto GL, Pinto L A, Cadaval RST (2019) Preparation of activated carbon from black wattle bark waste and its application for phenol adsorption. JOU ENV CHEM ENG 7(5), 103396. https://doi.org/10.1016/j.jece.2019.103396
Madadrang CJ, Kim HY, Gao G, Wang N, Zhu J, Feng H, Gorring M, Kasner ML, Hou S (2012) Adsorption behavior of EDTA-graphene oxide for Pb(II) removal. ACS Appl Mater Interfaces 4:1186–1193. https://doi.org/10.1021/am201645g
Maleki A, Pajootanb E, Hayati B (2015) Ethyl acrylate grafted chitosan for heavy metal removal from wastewater: equilibrium, kinetic and thermodynamic studies. J Taiwan Inst Chem E 51:127–134. https://doi.org/10.1016/j.jtice.2015.01.004
Mallekpour S, Madani M (2016) Functionalized-MnO2/chitosan nanocomposites: a promising adsorbent for the removal of lead ions. Carbohydr Polym 147:53–59. https://doi.org/10.1016/j.carbpol.2016.03.076
Man YB, Lei KM, Chow KL, Leung AOW, Mo WY, Wong MH (2020) Ecological risks of heavy metals/metalloid discharged from two sewage treatment works to Mai Po Ramsar site, South China. Environ Monit Assess 192(7):1–14. https://doi.org/10.1007/s10661-020-08397-w
Martín-Lara MA, Calero M, Ronda A, Rodriguez II, Escudero C (2020) Adsorptive behavior of an activated carbon for bisphenol A removal in single and binary (bisphenol A—heavy metal) solutions. Water 12(8):2150. https://doi.org/10.3390/w12082150
Monier M, Ayad DM, Wei Y, Sarhan AA (2010) Preparation and characterization of magnetic chelating resin based on chitosan for adsorption of Cu(II), Co(II), and Ni(II) ions. React Funct Polym 70:257–266. https://doi.org/10.1016/j.reactfunctpolym.2010.01.002
Mousavi SJ, Parvini M, Ghorbani M (2018) Experimental design data for the zinc ions adsorption based on mesoporous modified chitosan using central composite design method. Carbohydr Polym 188:197–212. https://doi.org/10.1016/j.carbpol.2018.01.105
Mozaffari M, Emami MRS, Binaeian E (2019) A novel thiosemicarbazide modified chitosan (TSFCS) for efficiency removal of Pb(II) and methyl red from aqueous solution. Int J Biol Macromol 123:457–467. https://doi.org/10.1016/j.ijbiomac.2018.11.106
Pandey S, Do JY, Kim J, Kang M (2019) Fast and highly efficient removal of dye from aqueous solution using natural locust bean gum based hydrogels as adsorbent. Int J Biol Macromol 143:60–75. https://doi.org/10.1016/j.ijbiomac.2019.12.002
Pang Y, Zeng G, Tang L, Zhang Y, Liu Y, Lei X, Li Z, Zhang J, Xie G (2011) PEI-grafted magnetic porous powder for highly effective adsorption of heavy metal ions. Desalination 281:278–284. https://doi.org/10.1016/j.desal.2011.08.001
Pauletto PS, Gonçalves JO, Pinto LAA, Dotto GL, Salau NPG (2020) Single and competitive dye adsorption onto chitosan–based hybrid hydrogels using artificial neural network modeling. J Colloid Interface Sci 560:722–729. https://doi.org/10.1016/j.jcis.2019.10.106
Petrella A, Spasiano D, Acquafredda P, De Vietro N, Ranieri E, Cosma P, Rizzi V, Petruzzelli V, Petruzzelli D (2018) Heavy metals retention (Pb(II), Cd(II), Ni(II)) from single and multimetal solutions by natural biosorbents from the olive oil milling operations. Process Saf Environ 114:79–90. https://doi.org/10.1016/j.psep.2017.12.010
Pooresmaeil M, Namazi H (2020) Application of polysaccharide-based hydrogels for water treatments. Hydrogels Based Nat Polymers 2020:411–455. https://doi.org/10.1016/B978-0-12-816421-1.00014-8
Rahman N, Nasir M (2020) Effective removal of acetaminophen from aqueous solution using Ca (II)-doped chitosan/β-cyclodextrin composite. J Mol Liq 301:112454. https://doi.org/10.1016/j.molliq.2020.112454
Ren H, Jiang J, Wu D, Gao Z, Sun Y, Luo C (2016) Selective adsorption of Pb(II) and Cr(VI) by surfactant-modified and unmodified natural zeolites: a comparative study on kinetics, equilibrium, and mechanism. Water Air Soil Pollut 227:101. https://doi.org/10.1007/s11270-016-2790-6
Repo E, Warchol JK, Kurniawan TA, Sillanpaa ME (2010) Adsorption of Co (II) and Ni (II) by EDTA- and/or DTPA-modified chitosan: kinetic and equilibrium modeling. Chem Eng J 161:73–82. https://doi.org/10.1016/j.cej.2010.04.030
Ritonga H, Nurfadillah A, Rembon FS, Ramadhan LOAN, Nurdin M (2019) Preparation of chitosan-EDTA hydrogel as soil conditioner for soybean plant (Glycine max). Groundw Sustain Dev 9:10027. https://doi.org/10.1016/j.gsd.2019.100277
Rout K, Mohapatra M, Mohapatra BK, Anand S (2009) Pb(II), Cd(II) and Zn(II) adsorption on low grade manganese ore. Int J Environ Sci Tech 1:106–122
Rout PR, Dash RR, Bhunia P (2016) Nutrient removal from binary aqueous phase by dolochar: highlighting optimization, single and binary adsorption isotherms and nutrient release. Process Saf Environ 100:91–107. https://doi.org/10.1016/j.psep.2016.01.001
Ruparelia JP, Duttagupta SP, Chatterjee AK, Mukherji S (2008) Potential of carbon nanomaterials for removal of heavy metals from water. Desalination 232:145–156. https://doi.org/10.1016/j.desal.2007.08.023
Sabrina FL, Andrei VI, Luana P, Guilherme LD, Luiz AAP, Tito RSC (2019) Preparation of activated carbon from black wattle bark waste and its application for phenol adsorption. J Environ Chem Eng 7(5):103396. https://doi.org/10.1016/j.jece.2019.103396
Shabalala AN, Ekolu SO, Diop S, Solomon F (2017) Pervious concrete reactive barrier for removal of heavy metals from acid mine drainage − column study. J Hazard Mater 323:641–653. https://doi.org/10.1016/j.jhazmat.2016.10.027
Shafiee M, Abedi MA, Abbasizadeh S, Sheshdeh RK, Mousavi SE, Shohani S (2020) Effect of zeolite hydroxyl active site distribution on adsorption of Pb (II) and Ni (II) pollutants from water system by polymeric nanofibers. Sep Sci Technol 55(11):1994–2011. https://doi.org/10.1080/01496395.2019.1624572
Silva SBD, Krolicka M, Broek LAM, Frissen AE, Boeriu CG (2018) Water-soluble chitosan derivatives and pH-responsive hydrogels by selective C-6 oxidation mediated by TEMPO - laccase redox system. Carbohydr Polym 186:299–309. https://doi.org/10.1016/j.carbpol.2018.01.050
Tabatabaeefar A, Yuan Q, Salehpour A, Hamane MR (2020) Batch adsorption of lead (ΙΙ) from aqueous solution onto novel polyoxyethylene sorbitan monooleate/ethyl cellulose microfiber adsorbent: kinetic, isotherm and thermodynamic studies. Sep Sci Technol J 55:5754. https://doi.org/10.1080/01496395.2019.1581218
Talebi M, Abbasizadeh S, Keshtkar AR (2017) Evaluation of single and simultaneous thorium and uranium sorption from water systems by an electrospun PVA/SA/PEO/HZSM5 nanofiber. Process Saf Environ 109:340–356. https://doi.org/10.1016/j.psep.2017.04.013
Wang H, Yuan X, Wu Y, Huang H, Zeng G, Liu Y, Wang X, Lin N, Qi Y (2013) Adsorption characteristics and behaviors of graphene oxide for Zn(II) removal from aqueous solution. Appl Surf Sci 279:432–440. https://doi.org/10.1016/j.apsusc.2013.04.133
Wang P, Cheng H, Ding J, Ma J, Jiang J, Huang Z, Li J, Pang S, Guan C, Gao Y (2020) Cadmium removal with thiosulfate/permanganate (TS/Mn(VII)) system: MnO2 adsorption and/or CdS formation. Chem Eng J 380:122585. https://doi.org/10.1016/j.cej.2019.122585
Wu D, Hu L, Wang Y, Wei Q, Yan L, Yan T, Li Y, Du B (2018) EDTA modified β cyclodextrin/chitosan for rapid removal of Pb(II) and acid red from aqueous solution. J Colloid Interface Sci 523:56–64. https://doi.org/10.1016/j.jcis.2018.03.080
Wu D, Wang Y, Li Y, Wei Q, Hu L, Yan T, Feng R, Yang L, Du B (2019) Phosphorylated chitosan/CoFe2O4 composite for the efficient removal of Pb(II) and Cd(II) from aqueous solution: adsorption performance and mechanism studies. J Mol Liq 277:181–188. https://doi.org/10.1016/j.molliq.2018.12.098
Xu Q, Wang Y, Jin L, Wang Y, Qin M (2017) Adsorption of Cu (II), Pb(II) and Cr (VI) from aqueous solutions using black wattle tannin immobilized nanocellulose. J Hazard Mater 331:91–99. https://doi.org/10.1016/j.jhazmat.2017.06.005
Zeng M, Zhang X, Shao L, Qi C, Zhang XM (2012) Highly porous chitosan microspheres supported palladium catalyst for coupling reactions in organic and aqueous solutions. J Organomet Chem 704:29–37. https://doi.org/10.1016/j.jorganchem.2012.01.003
Zeng D, Dai Y, Zhang Z, Wang Y, Cao X, Liu Y (2020) Magnetic solid-phase extraction of U (VI) in aqueous solution by Fe3O4@hydroxyapatite. J Radioanal Nucl Chem 324:1329–1337. https://doi.org/10.1007/s10967-020-07148-y
Zhao F, Repo E, Yin D, Meng Y, Jafari Sh, Sillanpää M (2015) EDTA-Cross-Linked β-Cyclodextrin: An Environmentally Friendly Bifunctional Adsorbent for Simultaneous Adsorption of Metals and Cationic Dyes. ENVIRON SCI TECHNOL 49(17): 10570–10580. https://doi.org/10.1021/acs.est.5b02227
Zhao D, Wang Z, Lu S, Shi X (2020) An amidoxime-functionalized polypropylene fiber: competitive removal of Cu(II), Pb(II) and Zn(II) from wastewater and subsequent sequestration in cement mortar. J Clean Prod 274:123049. https://doi.org/10.1016/j.jclepro.2020.123049
Author information
Authors and Affiliations
Contributions
This work was performed under the supervision of A. Parvareh and M. K. Moraveji. All authors contributed to the study conception and design the work. Material preparation, data acquisition, and analyses were performed by A. Panahandeh. Moreover, he was a major contributor in writing the first draft of the manuscript. A. Parvareh and M. K. Moraveji commented on previous versions of the manuscript. The final manuscript was read and approved by all authors. A draft version of the revised manuscript was performed by A. Panahandeh based on the reviewer’s comments. Final corrections on the revised manuscript as well as “answer to reviewers” was adjusted by A. Parvareh.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethical approval
Not applicable
Consent to participate
Not applicable
Consent to publish
Not applicable
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Panahandeh, A., Parvareh, A. & Moraveji, M.K. Synthesis and characterization of γ-MnO2/chitosan/Fe3O4 cross-linked with EDTA and the study of its efficiency for the elimination of zinc(II) and lead(II) from wastewater. Environ Sci Pollut Res 28, 9235–9254 (2021). https://doi.org/10.1007/s11356-020-11359-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11359-x