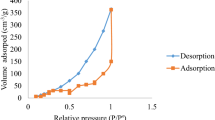
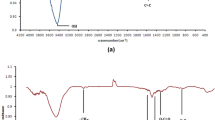
Abstract
In our research, the magnetic nanocomposite adsorbent Zn Fe3O4/SiO2 MCM-48 was prepared, and the ability of this nanocomposite to remove phosphate and nitrate ions from synthetic wastewater was investigated. Various batch adsorption conditions, including different pH, temperature, contact time, initial phosphate concentration and adsorbent dosage conditions, were considered. Phosphate and nitrate adsorption kinetics were well fitted by the pseudo-second-order kinetic model for all studied adsorbents. The adsorption process was represented by Langmuir isotherms. The thermodynamic parameters ∆G, ∆H and ∆S, which were determined using the Van't Hoff equation, indicated that the phosphate adsorption reactions on the Zn Fe3O4/SiO2 MCM-48 nanocomposite were spontaneous and endothermic in nature. The optimal conditions for the adsorption of phosphate and nitrate ions onto Zn Fe3O4/SiO2 MCM-48 were a pH of 2.0, temperature of 340 K and contact time of 66 min. The results presented here support the potential of using the Zn Fe3O4/SiO2 MCM-48magnetic nanocomposite as a material for the treatment of phosphate and nitrate ions in wastewater.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Alagha O., Manzar M. S., Zubair M., Anil I., Mu’azu N. D., & Qureshi A. (2020). Comparative adsorptive removal of phosphate and nitrate from wastewater using biochar-MgAl LDH nanocomposites: Coexisting anions effect and mechanistic studies. Nanomaterials (Basel), 10(2): 336. https://doi.org/10.3390/nano10020336
Afroze, S., & Sen, T. K. (2018). A review on heavy metal ions and dye adsorption from water by agricultural solid waste adsorbents. Water, Air, & Soil Pollution, 229(7), 225. https://doi.org/10.1007/s11270-018-3869-z.
Aljbour, S. H., Al-Harahsheh, A. M., Aliedeh, M. A., Al-Zboon, K., & Al-Harahsheh, S. (2016). Phosphate removal from aqueous solutions by using natural Jordanian zeolitic tuff. Adsorption Science & Technology, 35(3–4), 284–299. https://doi.org/10.1177/0263617416675176.
Aswin Kumar, I., & Viswanathan, N. (2019). Fabrication of zirconium(IV) cross-linked alginate/kaolin hybrid beads for nitrate and phosphate retention. Arabian Journal of Chemistry. https://doi.org/10.1016/j.arabjc.2019.06.006.
Bavaresco, J., Fink, J. R., Rodrigues, M. L. K., Gianello, C., BarrÓN, V., & Torrent, J. (2017). Chromium adsorption in different mineralogical fractions from subtropical soils. Pedosphere, 27(1), 106–111. https://doi.org/10.1016/S1002-0160(17)60300-X.
Berkessa, Y. W., Mereta, S. T., & Feyisa, F. F. (2019). Simultaneous removal of nitrate and phosphate from wastewater using solid waste from factory. Applied Water Science, 9(2), 28. https://doi.org/10.1007/s13201-019-0906-z.
Boopathy, R., Karthikeyan, S., Mandal, A. B., & Sekaran, G. (2013). Adsorption of ammonium ion by coconut shell-activated carbon from aqueous solution: Kinetic, isotherm, and thermodynamic studies. Environmental Science and Pollution Research, 20(1), 533–542. https://doi.org/10.1007/s11356-012-0911-3.
Dong, J., Du, Y., Duyu, R., Shang, Y., Zhang, S., & Han, R. (2019). Adsorption of copper ion from solution by polyethylenimine modified wheat straw. Bioresource Technology Reports, 6, 96–102. https://doi.org/10.1016/j.biteb.2019.02.011.
El-Naggar, I., Ahmed, S. A., Shehata, N., Sheneshen, E., Fathy, M., & Shehata, A. (2019). A novel approach for the removal of lead (II) ion from wastewater using Kaolinite/Smectite natural composite adsorbent. Applied Water Science, 9(1), 7.
Fathy, M., Abdel Moghny, T., Mousa, M. A., El-Bellihi, A.-H.A.-A., Awadallah, A. E. (2016). Absorption of calcium ions on oxidized graphene sheets and study its dynamic behavior by kinetic and isothermal models. Applied Nanoscience 1–13. https://doi.org/10.1007/s13204-016-0537-8
Fathy, M., Moghny, T. A., Mousa, M. A., Abdelraheem, O., Emam, A. A. (2019). Synthesis and study bromophenol blue dye adsorption efficiency of reduced graphene oxide produced by catalytic acid spray (CAS) method. Journal of the Australian Ceramic Society, 1–11
Fathy, M., Moghny, T. A., Mousa, M. A., El-Bellihi, A.-H. A., Awadallah, A. E. (2014). Sulfonated ion exchange polystyrene composite resin for calcium hardness removal.
Fathy, M., Zayed, M. A., & Moustafa, Y. (2019). Synthesis and applications of CaCO3/HPC core–shell composite subject to heavy metals adsorption processes. Heliyon, 5(8), e02215.
Gupta, M. D., Loganathan, P., & Vigneswaran, S. (2012). Adsorptive Removal of Nitrate and Phosphate from Water by a Purolite Ion Exchange Resin and Hydrous Ferric Oxide Columns in Series. Separation Science and Technology, 47(12), 1785–1792. https://doi.org/10.1080/01496395.2012.658487.
Gupta, N. K., Saifuddin, M., Kim, S., & Kim, K. S. (2020). Microscopic, spectroscopic, and experimental approach towards understanding the phosphate adsorption onto Zn–Fe layered double hydroxide. Journal of Molecular Liquids, 297, 111935. https://doi.org/10.1016/j.molliq.2019.111935.
Heba, H., El-Maghrabi, R. H., Ramzi, M., & Fathy, M. (2017). Novel mesoporous silica (MCM-41) and its characterization for oil adsorption from produced water injected in water injection projects using fixed bed column processes. Desalination and Water Treatment, 60, 70–77.
Hoque, M. A., Hassan, F. M., Seo, M.-H., Choi, J.-Y., Pritzker, M., Knights, S., et al. (2016). Optimization of sulfur-doped graphene as an emerging platinum nanowires support for oxygen reduction reaction. Nano Energy, 19, 27–38. https://doi.org/10.1016/j.nanoen.2015.11.004.
Hosny, R., Fathy, M., Ramzi, M., Moghny, T. A., Desouky, S., & Shama, S. (2016). Treatment of the oily produced water (OPW) using coagulant mixtures. Egyptian Journal of Petroleum, 25(3), 391–396.
Kazemi, E., Dadfarnia, S., & Haji Shabani, A. M. (2015). Dispersive solid phase microextraction with magnetic graphene oxide as the sorbent for separation and preconcentration of ultra-trace amounts of gold ions. Talanta, 141, 273–278. https://doi.org/10.1016/j.talanta.2015.04.024.
Keränen, A., Leiviskä, T., Hormi, O., & Tanskanen, J. (2015). Removal of nitrate by modified pine sawdust: Effects of temperature and co-existing anions. Journal of Environmental Management, 147, 46–54. https://doi.org/10.1016/j.jenvman.2014.09.006.
Khalid, A., Al-Juhani, A. A., Al-Hamouz, O. C., Laoui, T., Khan, Z., & Atieh, M. A. (2015). Preparation and properties of nanocomposite polysulfone/multi-walled carbon nanotubes membranes for desalination. Desalination, 367, 134–144. https://doi.org/10.1016/j.desal.2015.04.001.
Khedhri, I., Afli, A., & Aleya, L. (2017). Structuring factors of the spatio-temporal variability of macrozoobenthos assemblages in a southern Mediterranean lagoon: How useful for bioindication is a multi-biotic indices approach? Marine Pollution Bulletin, 114(1), 515–527. https://doi.org/10.1016/j.marpolbul.2016.10.023.
Kilpimaa, S., Runtti, H., Kangas, T., Lassi, U., & Kuokkanen, T. (2014). Removal of phosphate and nitrate over a modified carbon residue from biomass gasification. Chemical Engineering Research and Design, 92(10), 1923–1933. https://doi.org/10.1016/j.cherd.2014.03.019.
Korzeniowska, A., Grzybek, J., Kałahurska, K., Kubu, M., Roth, W. J., & Gil, B. (2019). The structure-catalytic activity relationship for the transient layered zeolite MCM-56 with MWW topology. Catalysis Today. https://doi.org/10.1016/j.cattod.2019.09.044.
Liu, M., Hou, L.-a, Yu, S., Xi, B., Zhao, Y., & Xia, X. (2013). MCM-41 impregnated with A zeolite precursor: Synthesis, characterization and tetracycline antibiotics removal from aqueous solution. Chemical Engineering Journal, 223, 678–687. https://doi.org/10.1016/j.cej.2013.02.088.
Mahmoud Fathy, T. A. M., Mousa, M. A., ElBellihi, A.-H.-A., & Awadallah, A. E. (2015). Sulfonated ion exchange polystyrene composite resin for calcium hardness removal. International Journal of Emerging Technology and Advanced Engineering, 5(10), 20–29.
Mamba, G., & Mishra, A. K. (2016). Graphitic carbon nitride (g-C3N4) nanocomposites: A new and exciting generation of visible light driven photocatalysts for environmental pollution remediation. Applied Catalysis B: Environmental, 198, 347–377. https://doi.org/10.1016/j.apcatb.2016.05.052.
Qiao, H., Mei, L., Chen, G., Liu, H., Peng, C., Ke, F., et al. (2019). Adsorption of nitrate and phosphate from aqueous solution using amine cross-linked tea wastes. Applied Surface Science, 483, 114–122. https://doi.org/10.1016/j.apsusc.2019.03.147.
Sakate, S. S., Shinde, S. H., Kasar, G. B., Chikate, R. C., & Rode, C. V. (2018). Cascade synthesis of dihydrobenzofuran via Claisen rearrangement of allyl aryl ethers using FeCl3/MCM-41 catalyst. Journal of Saudi Chemical Society, 22(4), 396–404. https://doi.org/10.1016/j.jscs.2017.08.006.
Gupta, S. S., Ton, V.-K., Beaudry, V., Rulli, S., Cunningham, K., & Rao, R. (2003). Antifungal activity of amiodarone is mediated by disruption of calcium homeostasis. Journal of Biological Chemistry, 278(31), 28831−28839
Zhang, Q., He, M., Chen, B., & Hu, B. (2016a). Preparation, characterization and application of Saussurea tridactyla Sch-Bip as green adsorbents for preconcentration of rare earth elements in environmental water samples. Spectrochimica Acta Part B: Atomic Spectroscopy, 121, 1–10. https://doi.org/10.1016/j.sab.2016.04.005.
Zhang, Y., Huang, Y., Wang, X., Guo, Y., Jia, D., Tang, X. (2016b). Improved electrochemical performance of lithium iron phosphate in situ coated with hierarchical porous nitrogen-doped graphene-like membrane. Journal of Power Sources, 305, 122–127. https://doi.org/10.1016/j.jpowsour.2015.11.092
Zhang, Y., Si, L., Zhou, B., Zhao, B., Zhu, Y., Zhu, L., & Jiang, X. (2016c). Synthesis of novel graphene oxide/pristine graphene/polyaniline ternary composites and application to supercapacitor. Chemical Engineering Journal, 288, 689–700. https://doi.org/10.1016/j.cej.2015.12.058.
Zhang, X.-Z., Zhou, Y., Zhang, W., Zhang, Y., & Gu, N. (2016d). Polystyrene@Au@prussian blue nanocomposites with enzyme-like activity and their application in glucose detection. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 490, 291–299. https://doi.org/10.1016/j.colsurfa.2015.11.035.
Zhang, Y., Zhang, Y., Huang, L., Zhou, Z., Wang, J., Liu, H., & Wu, H. (2016e). Hierarchical carambola-like Li4Ti5O12-TiO2 composites as advanced anode materials for lithium-ion batteries. Electrochimica Acta, 195, 124–133. https://doi.org/10.1016/j.electacta.2016.02.092.
Zhang, Z., Zhai, S., Wang, M., Ji, H., He, L., Ye, C., et al. (2016f). Photocatalytic degradation of rhodamine B by using a nanocomposite of cuprous oxide, three-dimensional reduced graphene oxide, and nanochitosan prepared via one-pot synthesis. Journal of Alloys and Compounds, 659, 101–111. https://doi.org/10.1016/j.jallcom.2015.11.027.
Zhang, Z., Zhang, H., Zhu, L., Zhang, Q., & Zhu, W. (2016g). Hierarchical porous Ca(BO2)2 microspheres: Hydrothermal–thermal conversion synthesis and their applications in heavy metal ions adsorption and solvent-free oxidation of benzyl alcohol. Chemical Engineering Journal, 283, 1273–1284. https://doi.org/10.1016/j.cej.2015.08.073.
Zhao, Y., Zhang, Z., Dai, L., Mao, H., & Zhang, S. (2017). Enhanced both water flux and salt rejection of reverse osmosis membrane through combining isophthaloyl dichloride with biphenyl tetraacyl chloride as organic phase monomer for seawater desalination. Journal of Membrane Science, 522, 175–182. https://doi.org/10.1016/j.memsci.2016.09.022.
Zikos, D., Hagedorn, K. (2017) Competition for water resources from the European perspective A2—Ziolkowska, Jadwiga, R. In: J. M. Peterson (ed.) Competition for Water Resources (pp 19–35). Elsevier. https://doi.org/10.1016/B978-0-12-803237-4.00002-1.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Mubarak, M.F., Hosny, R. (2021). Comparative Analysis on Adsorption Properties and Mechanisms of Nitrate and Phosphate Ions by a Zn Fe3O4/SiO2 MCM-48 Magnetic Composite: Kinetic and Isotherm Studies. In: Al-Maktoumi, A., et al. Water Resources in Arid Lands: Management and Sustainability. Advances in Science, Technology & Innovation. Springer, Cham. https://doi.org/10.1007/978-3-030-67028-3_21
Download citation
DOI: https://doi.org/10.1007/978-3-030-67028-3_21
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-67027-6
Online ISBN: 978-3-030-67028-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)