Abstract
The unsustainable settlement and high industrialization around the catchment of the Baltic Sea has left records of anthropogenic heavy metal contamination in Baltic Sea sediments. Here, we show that sediments record post-industrial and anthropogenic loads of Cd, Zn, and Pb over a large spatial scale in the Baltic Sea. We also demonstrate that there is a control on the accumulation of these metals in relation to oxic/anoxic conditions of bottom waters. The total concentrations of Cd, Zn, and Pb were obtained with the near-total digestion method in thirteen cores collected from the Bothnian Bay, the Bothnian Sea, and the west and central Baltic Proper. The lowest average concentrations of Cd, Zn, and Pb were observed in Bothnian Bay (0.4, 125, 40.2 mg kg−1 DW, respectively). In contrast, the highest concentrations were observed in the west Baltic Proper (5.5, 435, and 56.6 mg kg−1 DW, respectively). The results indicate an increasing trend for Cd, Zn, and Pb from the early nineteenth century until the 1970s, followed by a decrease until 2000–2008. However, surface sediments still have concentrations above the pre-industrial values suggested by the Swedish EPA (Cd is 0.2, Zn is 85, and Pb is 31 mg kg−1 DW). The results also show that the pre-industrial Cd, Zn, and Pb concentrations obtained from 3 cores with ages < 1500 B.C. were 1.8, 1.7, and 1.2 times higher, respectively, than the pre-industrial values suggested by the Swedish EPA. To conclude, accumulations of metals in the Baltic Sea are governed by anthropogenic load and the redox conditions of the environment. The significance of correct environmental governance (measures) can be illustrated with the reduction in the pollution of Pb, Zn, and Cd within the Baltic Sea since the 1980s.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Anthropogenic metal inputs are reported from seas and coastlines worldwide, e.g., in the East China Sea (Daoji and Daler 2004), Caspian Sea (Bastami et al. 2015; Abadi et al. 2019), Yumurtalik coast (Aytekin et al. 2019), North-East Atlantic (Rodrigues et al. 2009), Greenland Sea (Neff 2002a), North Sea, Mediterranean, Black Sea, and Baltic Sea (Tornero and Hanke 2016). Heavy metals can be toxic to humans/animals and are incorporated into the sea food web through water, food, and sediments (Neff 2002a; Tchounwou et al. 2012; Aytekin et al. 2019). Therefore, heavy metals such as cadmium (Cd), lead (Pb), and zinc (Zn) are frequently studied and monitored to avoid negative impacts on society (Skerfving et al. 1999; Tchounwou et al. 2012) and ecosystems (Neff 2002a; b; c; Aytekin et al. 2019). In Northern Europe, the unsustainable settlement in the catchment of the Baltic Sea region has turned this waterbody into one of the most polluted seas and dead zones in the world (Borg and Jonsson 1996; Conley et al. 2002; Carstensen et al. 2014a). With noticeable pollution in the past 50 years, the 1974 European Regional Sea Conventions (Tornero and Hanke 2016), specifically the HELCOM convention, have taken several actions. These actions have been taken to protect and reduce the pollution of the Baltic Sea (HELCOM 2018), including pollution by heavy metals.
Two common heavy metals associated with health risks in the Baltic Sea region are Pb and Cd. Both metals are reported to cause severe damage to organs (kidney), and Pb also causes neurobehavioral and neurophysiological disease (Skerfving et al. 1999). In contrast, Zn is an essential element for humans and animals (Remeikaitė-Nikienė et al. 2018). However, under long-term high exposure conditions, Zn might cause iron/copper deficiency and neuron-related illness (Ciubotariu et al. 2015). In addition, when Zn is compared to Cd and Pb, it shows a higher affinity to accumulate in marine organisms and tissues (Aytekin et al. 2019). According to the Swedish Environmental Protection Agency (EPA), the pre-industrial values for coastal and sea sediments are based on pre-industrial observations, with values of 0.2, 85, and 31 mg kg−1 dry weight (DW) for Cd, Zn, and Pb, respectively (Swedish EPA 2000) (Appendix Table 7). Cadmium, Zn, and Pb concentrations above these values have been reported in several studies focusing on separate parts of the Baltic Sea, Bothnian Bay (Leivuori and Niemistö 1995; Perttilä 2003; Vallius 2014), Gulf of Finland (Vallius 2014), and Gdansk and Baltic Proper (Suess and Erlenkeuser 1975; Perttilä 2003; Zaborska 2014). In addition, previous studies reported maximum concentrations of Cd, Zn, and Pb in the 1970s (Leivuori and Niemistö 1995; Borg and Jonsson 1996; Zaborska 2014).
Studies have reported the effects of climate change, the long-term tendency (early twentieth century) of increasing anoxic areas of bottom waters, and the excessive anthropogenic nutrient/organic loads in the deep and central parts of the Baltic Sea (Borg and Jonsson 1996; Conley et al. 2002; Kabel et al. 2012; Carstensen et al. 2014a; b; Mohrholz 2018). These imposed changes in redox conditions influence the biogeochemical transformations of the suspended particulate matter (SPM) (Beldowski et al. 2010), colloids (Neff 2002b), and soluble metal forms within the water column and sediments (Borg and Jonsson 1996; Neff 2002a; b; c). In addition, metal concentrations in sediments can correlate with grain size surface charge density, cation exchange capacity (CEC), and specific surface area (SSA) (Neff 2002b; Fukue et al. 2006). Anoxic conditions release adsorbed metals from the surface of iron (Fe)/manganese (Mn) hydroxides, and they instead form metal sulfides (Neff 2002c; Beldowski et al. 2010). However, contrary to the assumptions of Borg and Jonsson (1996) and Rogan Šmuc et al. (2018) report higher concentrations of Pb from reduced (anoxic) sediments related to the formation of stable Fe and Mn complexes. Cadmium and Zn are also under the influence of oxic/anoxic conditions (Pohl and Hennings 1999; Neff 2002a; b). Under oxic conditions, Cd is released when organic matter (OM) is mineralized (Neff 2002a; Rogan Šmuc et al. 2018), while Zn is adsorbed to Fe/Mn oxides and OM (Neff 2002b). Overall, Cd, Zn, and Pb are reported to be more abundant in oxic than anoxic waters (Öztürk 1995; Pohl and Hennings 1999); additionally, the foremost soluble Cd, Zn, and Pb species in anoxic waters originate from the bisulfide complexes of these metals (Öztürk 1995; Neff 2002c).
In this report, the overall aim was to study Cd, Zn, and Pb metal pollution in the sediments of the Baltic Sea, focusing on anomalies in the different basins of the Baltic Sea using the same field and analytical methods. This approach will enable us to have a nonbiased comparison among different basins of the Baltic Sea. A specific aim was to identify anthropogenic loads and understand the processes that control the quantity and distribution of Cd, Pb, and Zn in sediments of the Gulf of Bothnia (Bothnian Bay and Bothnian Sea), Åland Sea, and Baltic Proper. Furthermore, the effects of oxic/anoxic conditions in the bottom waters of the Baltic Sea and their relation to observed metal concentrations in sediments are studied. In addition, this study will compare the obtained results of pre-industrial Cd, Zn, and Pb from the aforementioned basins to the pre-industrial values published by the Swedish EPA (Swedish EPA 2000). The study of metal concentration trends will further assist us in evaluating how effective measures to reduce concentrations are over time. Overall, we expect to contribute to the understanding of Cd, Zn, and Pb in sediments under oxic/anoxic conditions, which is important with regard to future climate change- and eutrophication-related anoxic growth in the Baltic Sea and similar water bodies.
Methods
A total of 137 sediment samples were obtained during the Swedish Environmental Monitoring Programme of offshore sediments in 2008, which was operated by the Geological Survey of Sweden on behalf of the Swedish Environmental Protection Agency (Apler and Josefsson 2016). The collected cores were from 13 stations, including two in Bothnian Bay (stations 17 and 1), three in the Bothnian Sea and Åland Sea (stations 2–4), three in the central Baltic Proper (stations 5–7), and five in the west Baltic Proper (stations 8–12) (Apler and Josefsson 2016) (Fig. 1 and Table 1). Sediment cores were taken with a Gemini gravity corer (Niemistö 1974) onboard the S/V Ocean Surveyor vessel between May and August 2008 (Apler and Josefsson 2016). Cores were sliced into 1-cm intervals (Appendix Tables 2, 3, 4, and 5), freeze-dried, and ground by mortar and pestle. Sediment accumulation rates and the age of each slice were estimated (Appendix Tables 2, 3, 4, and 5) from Cesium-137 determination in sediments (Josefsson and Apler 2019). However, not all slices could be included in this study due to insufficient sample weights for total metal analysis (Table 1 and Appendix Tables 2, 3, 4, and 5).
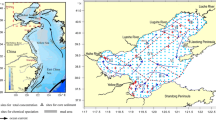
Map of the Baltic Sea showing the location of the 13 cores (numbered 1–12 and 17) used in this study. Image processed and adapted under GNU Free Documentation License. Image is georeferenced by ArcGIS. The graticule lines, north direction, station locations, and name of countries and seas are added to Original Image. In addition, color of lands and borderlines are modified
All 137 samples were analyzed for the total concentrations of Cd, Zn, and Pb. Sample preparation included digestion of a 250-mg dry weight (DW) sample with stepwise addition of hydrofluoric, nitric, and perchloric acids. The obtained solution was dried until incipient dryness was achieved, and subsequently, the solution phase was retained by the addition of aqua regia. Metal concentrations were determined by inductively coupled plasma mass spectrometry at Activation Laboratories Ltd. (ActLabs), Canada (http://www.actlabs.com/, Appendix Table 6). The precision of the analytical method was controlled with the coefficient of variation (Appendix Table 6) using anonymously randomized duplicates (Gill 2014).
The calculation of pre-industrial levels of metals in sediments was based on the quality and quantity of data (Gałuszka and Migaszewski 2011; Zgłobicki et al. 2011; de Paula Filho et al. 2015). The direct method, also known as the geochemical method, is most frequently used (Gałuszka and Migaszewski 2011; Zgłobicki et al. 2011; de Paula Filho et al. 2015) and was thus used in this study. This method is based on visualization and relies on samples that date back to (i.e., are older than) the industrial revolution and are thus largely free of human influence (Zgłobicki et al. 2011). Visually, the quasi-linear, invariable concentration trend with depth in old profiles was recognized (Gałuszka and Migaszewski 2011; Zgłobicki et al. 2011). Pre-industrial metal median concentration (Gałuszka and Migaszewski 2011) values were considered in the three cores (stations 7, 9, and 10 located in the Baltic Proper) with the lowest sedimentation rates (< 0.08 cm/year), which contained sediments > 500 years of age (Fig. 2, Appendix Tables 4 and 5). ArcGIS was used to georeference and plot sampling points in the Baltic Sea image. In addition, the images were developed/edited in Affinity Designer Software version 1.7.3.481. The graphs and statistical analysis were created and conducted in R Studio (2018).
Concentration (mg kg−1 DW) profiles for Cd, Zn, and Pb in the three cores with the lowest sediment accumulation rates which were selected for determination of pre-industrial concentrations. Numbers on the figures are the calculated depositional years of the sediment slice at that depth, based on dating using Caesium-137. See Fig. 1 and Table 1 for core location
Results
Pre-industrial Cd, Zn, and Pb concentrations
The median and standard deviation (SD) of Cd, Zn, and Pb concentrations from sediments > 500 years old are 0.25 (0.21), 141.5 (9.05), and 36.3 (6.23) mg kg−1 DW, respectively (Fig. 2, and Appendix Tables 4, 5, and 7). These values are based on cores located in the Baltic Proper, areas where low sediment accumulation rates allow the sampler to reach sufficiently old samples in the sediment. These calculated pre-industrial concentrations are 1.25, 1.7, and 1.2 times, respectively, higher than the Cd, Zn, and Pb pre-industrial values suggested by the Swedish Environmental Protection Agency (Swedish EPA 2000) (Appendix Table 7).
Anthropogenic loads of Cd, Zn, and Pb
Bothnian Bay
The average surface sample (0–1 cm) concentrations in cores 17 and 1, collected in northern and southern Bothnian Bay, exceed the calculated pre-industrial values by 0.9, 52.5, and 32.7 mg kg−1 DW for Cd, Zn, and Pb, respectively. At station 17, the concentration peaks correspond to the early ca. 1980s and are as high as 1.1, 176, and 78.2 mg kg−1 DW for Cd, Zn, and Pb, respectively. The sediment accumulation rates of stations 17 and 1 are similar; however, at station 1, Cd and Zn continue to increase and are highest in the surface sample, corresponding to ca. year 2000, with values of 1.3 and 217 mg kg−1 DW, respectively. In contrast, the concentrations of Pb at station 1 decrease from 124 mg kg−1 DW in the samples from ca. 1980s to 91 mg kg−1 DW in the topmost sample corresponding to ca. 2000.
The Bothnian Sea and Åland Sea
The average surface sample (0–1 cm) concentrations in cores 2, 3, and 4 collected in the northern and southern Bothnian Sea and the Åland Sea exceed the calculated pre-industry values by 0.1, 31.5, and 2 mg kg−1 DW for Cd, Zn, and Pb, respectively. Below this depth, in the top 10 cm and towards the surface, the concentrations decreased, although it should be noted that at station 2, only one sample was analyzed from these depths, which makes the trend uncertain (Fig. 4). All three stations peak at depths of 10–20 cm, corresponding roughly to the early 1980s (Fig. 4 and Appendix Table 3). The Cd concentrations decreased strongly below a peak at 26–27 cm at station 2 (1.1 mg kg−1 DW). The corresponding pattern for Zn was similar between stations 2 and 4, while concentrations were lower at station 3; however, for Pb, station 4 generally had the highest concentration (Fig. 4).
Central Baltic Proper
The average surface concentrations (0–1 cm) in cores 5–7 collected in the central Baltic Proper exceed the calculated pre-industrial values by 3.6 and 710 mg kg−1 DW for Cd and Zn, respectively. However, the average Pb concentrations from the surface samples (Fig. 5) were lower than the calculated pre-industrial value of 4.9 mg kg−1 DW. In stations of the central Baltic Proper, the sediment accumulation rates were highly variable and decreased in the order of stations 5 > 6 > 7 (Fig. 5 and Appendix Table 4). All three cores showed a similar trend of strongly decreasing metal concentrations towards the surface. At stations 6 and 7, this decrease is observed from the nineteenth century onward, and the decrease was observed at station 5 from an undefined age. At station 5, as the concentrations increased to the bottom of the core, the maximum values may not have been reached due to high sediment accumulation rates. The metal concentrations reached high values in all cores, up to approximately 10–15 mg kg−1 DW Cd, 700–800 mg kg−1 DW Zn, and 100–120 mg kg−1 DW Pb (Fig. 5 and Appendix Table 5). The highest Cd and Zn concentrations occurred at station 5, with values as high as 14.7 and 819 mg kg−1 DW, respectively, in approximately the 1950s. The highest Pb concentration occurred at station 7, with a value as high as 128 mg kg−1 DW, before 1895.
West Baltic Proper
The average surface concentrations (0–1 cm) in cores 8–12 collected in the west Baltic Proper exceed the calculated pre-industrial values by 2.4, 112, and 114.5 mg kg−1 DW for Cd, Zn, and Pb, respectively. Stations 8–12 show a decreasing concentration trend in the surface samples, although the values still exceed the calculated pre-industrial values at most stations (Fig. 6a, b and Appendix Table 7). For stations 9, 11, and 12, the highest concentrations are present in the sediment layer sampled below the surface layer, and no long-term decreasing trend is thus apparent. For stations 8 and 10, the peak concentrations are further down in the core. The metal concentrations reached high values in all cores, up to approximately 10 mg kg−1 DW Cd, 700–800 mg kg−1 DW Zn, and 100–120 mg kg-1 DW Pb (Fig. 6a, b and Appendix Table 5). The highest Cd concentrations occurred at station 8, with a value as high as 10.4 mg kg−1 DW in approximately the early 1970s. The highest Zn and Pb concentrations occurred at stations 9 and 12, respectively, with maximum values of 753 mg kg−1 DW and 114 mg kg−1 DW, respectively, in approximately the mid-1970s.
Discussion
Pre-industrial values
Differences between the calculated pre-industrial concentrations for Cd, Pb, and Zn in this study and the Swedish EPA (Swedish EPA 2000) show the complex geochemistry of marine sediments (Reimann and Garrett 2005; Gałuszka and Migaszewski 2011). It is important to note that the pre-industrial values established by the Swedish EPA are median values for the entire marine environment of Sweden and thus do not take any regional differences in, e.g., bedrock chemistry or redox conditions, into account. Fukue et al. (2006) report anomalies in pre-industrial metal concentrations related to sorption/adsorption capacity and the characteristics of the specific sediment sample. Additionally, pre-industrial Zn and Pb concentrations show different values when compared to gneiss bedrock (Fukue et al. 2006). The highest concentrations of Zn are also visible in the longer cores, which might be an indicator of the dominant feature of the bedrock (Fukue et al. 2006) and the possible presence of Zn within heavy minerals (Neff 2002b). Fukue et al. (2006) also consider grain size, CEC and SSA as factors affecting the observed concentration anomalies from pre-industrial values in sediment cores. In this study, samples with Cd, Zn, and Pb concentrations lower (Fig. 3) than the calculated pre-industrial values for the Baltic Proper cores with an age < 1500 B.C. (Fig. 2) might be explained by the difference in the texture, bedrock, CEC, SSA (Fukue et al. 2006), and oxic/anoxic conditions (Carstensen et al. 2014a).
Concentration (mg kg−1 DW) profiles of Cd, Zn, and Pb from stations 17 and 1 in the North and South of the Bothnian Bay. Indicated ages are calculated from sediment accumulation rates based on Caesium-137 concentrations in sediments. Median calculated background values obtained for cores 7, 9, and 10 are plotted for reference (dashed vertical lines). See Fig. 1 and Table 1 for core location
Declining trends towards the surface
In this study, peak concentrations are mainly found around the 1970s and 1980s (with the exception of stations 6 and 7, see Fig. 5). Borg and Jonsson (1996) also reported elevated concentrations of Cd, Zn, and Pb from Baltic Sea sediments dating back to 1970 and later. However, most of the studied locations show a recovery trend after the 1980s, which aligns with the HELCOM reports, suggesting the efficiency of policies limiting emissions of Cd, Zn, and Pb to the environment. This result means that the load and concentrations of pollutants previously introduced in the Baltic Sea from industry and agriculture have been reduced. In the case of Pb, the general decreasing trend starting in the 1980s is attributed to restrictions of Pb-based additives in fuels (Östlund and Sternbeck 2001; Beldowski et al. 2010; Vallius 2014).
All cores from the Gulf of Bothnia and Åland Sea (Figs. 3 and 4) show lower maximum concentrations of Cd, Zn, and Pb than cores from the Baltic Proper (Figs. 5 and 6). Comparing Cd, Zn, and Pb among different basins, the west and central Baltic Proper show higher peaks and concentrations (Fig. 4). These findings agree with previous observations of higher Cd, Zn, and Pb concentrations in the Baltic Proper relative to those in the entire Baltic Sea (Suess and Erlenkeuser 1975; Perttilä 2003; Zaborska 2014). The strong reducing conditions near the sediment-water interface (Carstensen et al. 2014a) might explain the fixation of metal species in anoxic sediments (Borg and Jonsson 1996).
Concentration (mg kg−1 DW) profiles of Cd, Zn, and Pb from stations 2–4 belonging to the North and South of the Bothnian Bay and Åland Sea are presented. Indicated ages are calculated from sediment accumulation rates based on Caesium-137 concentrations in sediments. Median calculated background values obtained for cores 7, 9, and 10 are plotted for reference (dashed vertical lines). See Fig. 1 and Table 1 for core location
Concentration (mg kg−1 DW) profiles of Cd, Zn, and Pb from stations 5–7 belonging to the central Baltic Proper are presented as Central Baltic Proper. Indicated ages are calculated from sediment accumulation rates based on Caesium-137 concentrations in sediments. Median calculated background values obtained for cores 7, 9, and 10 are plotted for reference (dashed vertical lines). See Fig. 1 and Table 1 for core location
Concentration (mg kg−1 DW) profiles of Cd, Zn, and Pb from stations 8–10 (a) and 11–12 (b) belonging to the central Baltic and west Baltic Proper. Indicated ages are calculated from sediment accumulation rates based on Caesium-137 concentrations in sediments. Median calculated background values obtained for cores 7, 9, and 10 are plotted for reference (dashed vertical lines). See Fig. 1 and Table 1 for core location
Illustrating the expected behavior of Cd, Zn, and Pb in the Baltic Sea water, pore water, sediments (Mn precipitates, sulfides), and OM under oxic (a, b) and anoxic conditions (c, d). Different metals behave in certain ways from dissolution in oxic and anoxic waters (a, c) and formation of hydroxides in oxic water (a, b) to precipitation and interacting with sulfides in anoxic water (d). Higher concentrations of Cd, Zn, and Pb are present in oxic waters. When oxygen and OM are available, OM mineralization release Cd into solution (a) and at bottom sediments the soluble Cd in the pore water interacts with Fe/Mn precipitates (b). However, under the same conditions, Zn is bound to OM (a) and Pb is attracted towards Mn precipitates in solution (a). At the bottom sediments, OM is degraded over time and Zn is released and associated with Mn/Fe oxyhydroxides (b). Also, the flux between sediments and the water column tends to be higher for Zn and lower for Pb (b). In the anoxic water, in general lower concentrations of Cd, Zn, and Pb are present in solution (c) owing its solubility to bisulfides. In the anoxic bottom sediments, Cd and Zn have high affinity towards sulfides in clay-rich sediments and Pb reacts with Mn/Fe forming complexes. The flux from sediment to the water column is faster for Zn and slower for Pb in anoxic waters (d). Image is developed in Affinity Designer Software version 1.7.3.481. References (Öztürk 1995; Borg and Jonsson 1996; Pohl and Hennings 1999; Neff 2002a; b; c; Ingri et al. 2014; Rogan Šmuc et al. 2018; Beldowski et al. 2010).
Gulf of Bothnia
Concerning the general behaviors of Cd, Zn, and Pb in the Gulf of Bothnia (Figs. 3 and 4), the results from this study and previous reports (Leivuori and Niemistö 1995; Vallius 2014; Zaborska 2014) show similar patterns between profiles. Cadmium is reported to be as high as 0.2–2 mg kg−1 DW, Zn ranged from 60 to 220 mg kg−1 DW, and Pb ranged from 5 to 200 mg kg−1 DW (Leivuori and Niemistö 1995; Borg and Jonsson 1996; Perttilä 2003; Vallius 2014), which are in the same range as Pb in this study (Cd 0.1–1.3 mg kg−1 DW, Zn 81–242 mg kg−1 DW, and Pb 16–124 mg kg−1 DW). On average, the water depth is deeper in the Bothnian Sea (65 m) than in Bothnian Bay (40 m) (Håkansson et al. 1996). In addition, the freshwater load and water circulation are higher in Bothnian Bay than in the Bothnian Sea (Håkansson et al. 1996; Perttilä 2003), which oxygenates the bottom waters and brings metals into solution. Overall, Cd, Zn, and Pb are reported to be more abundant in oxic waters than in anoxic waters (Fig. 7a) (Öztürk 1995; Pohl and Hennings 1999). In the case of the Bothnian Sea and Åland Sea, the Cd and Zn concentrations are higher at stations 2 and 4 than at station 1. Stations 2 and 4 show similar concentration anomalies and peak concentrations of Cd and Zn. This result might be an effect of anoxic deep water at stations 2 and 4, which are located at water depths of 200 and 230 m, respectively. However, with shallower and better freshwater circulation in the Gulf of Bothnia, this concentration does not exceed the peak concentrations in the Baltic Proper cores. In addition, the dynamics of the Gulf of Bothnia might allow coarser particles with low CEC and SSA (Radzevièius 2002) to enter the basin and therefore restrict the adsorption of soluble metals (Jönsson et al. 2005). With available metals and previously found ferrohydrate nodules in the southern Bothnian Sea (Borg and Jonsson 1996), which act as adsorption surfaces for soluble metals, higher concentrations of sediment metals are expected in the Bothnian Sea. Increases in Cd concentrations in surface samples under oxic conditions might be explained by OM mineralization (Rogan Šmuc et al. 2018), which releases soluble Cd from the pore water (Rogan Šmuc et al. 2018) that can be transferred to the surface (Rogan Šmuc et al. 2018). With soluble Cd and the presence of Mn precipitates (Pohl and Hennings 1999) and ferrohydrate nodules (Borg and Jonsson 1996), Cd enrichment might occur in the surface layers. Zinc in the water column has a higher affinity to form stable colloidal OM; this colloidal OM has stronger bonds than those of Zn adsorbed to SPM (Neff 2002b). In oxic sediments, OM and Fe/Mn oxides are associated with higher Zn concentrations in bottom sediments (Neff 2002b; Ingri et al. 2014). Moreover, high Zn concentrations are found in phytoplankton, which affects the Zn concentrations of bottom sediments (Ingri et al. 2014) through the degradation of OM followed by Fe/Mn oxide or ferrohydrate nodule uptakes (Fig. 7a). The lower concentrations of Zn in the top layers of oxic sediments (Figs. 3 and 4) could be explained by the faster flux of dissolved Zn from sediments into the oxic water column (Neff 2002b) (Fig. 7b). For lead, close contamination sources and the inland transport of contamination into the Bothnian Bay basin may have caused the higher concentrations reported by Perttilä (2003) than those found in this study. Lead concentrations in the SPM of oxic/anoxic regions are reported to be in the same range as the concentrations of Zn (Öztürk 1995). Additionally, Pb in oxic sediments is reported to be associated with oxic Fe/Mn coatings loaded on clay particles (Neff 2002c). Moreover, the surface concentrations of Cd, Zn, and Pb at station 1 are higher than those in the other cores of the Gulf of Bothnia. Higher surface sample concentrations at this station might be due to high organic matter inputs and direct metal loads from the smelter plant located at Rönnskär, which is close to station 1 (HELCOM 2013), which has been an important pollution source. However, there are industrial source contamination hotspots reported close to all five cores (17 and 1–4) (HELCOM 2013). Stations 17, 1, and 4 might have experienced pollution from the main marine navigation routes (HELCOM 2018) as well as from all main industrial pollution sources (HELCOM 2013). The elevated concentrations of Cd, Pb, and Zn in the Åland Sea core (Fig. 3) can be related to agricultural runoff hotspot pollution (HELCOM 2013) and the high-density traffic line connecting the Gulf of Bothnia and the Baltic Proper (HELCOM 2018).
Baltic Proper
Concerning the general behavior of Cd, Zn, and Pb in the Baltic Proper, the comparison of metals in the different sectors of the Baltic Proper (Figs. 5 and 6) shows similar patterns in this and previous studies (Leivuori and Niemistö 1995; Zaborska 2014). Cadmium is reported to be as high as 0.1–10.93 kg−1 DW, Zn as high as 44–1021 kg−1 DW, and Pb as high as 18–> 200 mg kg−1 DW (Suess and Erlenkeuser 1975; Perttilä 2003; Zaborska 2014), which are similar to the values in this report (Cd 0.1–14.1 mg kg−1 DW, Zn 87–816 mg kg−1 DW, and Pb 20–128 mg kg−1 DW). In general, the high concentrations of metals in the Baltic Proper might be explained by the high organic matter content in sediments, the presence of sulfides in anoxic environments (Borg and Jonsson 1996), and the relatively longer residence time of anoxic waters (Leivuori and Niemistö 1995). Kabel et al. (2012) also suggest that the anoxic waters of the Baltic Proper can become more widespread with current and future climate change. As mentioned, Cd, Zn, and Pb are influenced by anoxic environments (Fig. 7c). The foremost soluble Cd, Zn, and Pb species in anoxic waters originate from the bisulfide complexes of these metals (Fig. 7c) (Öztürk 1995; Neff 2002c). The higher concentrations of Cd in the Baltic Proper might be related to the available fine particles and clay-rich sediments in deep anoxic waters (Pohl and Hennings 1999). Pohl and Hennings (1999) reported the high affinity of Cd sulfide species to these particles (Fig. 7d). In reduced sediments, Zn has a higher affinity towards OM and sulfides than towards Fe/Mn oxides (Neff 2002b; Ingri et al. 2014). This feature could also be observed in pore waters of anoxic sediments where soluble Zn adsorbs to OM (Neff 2002b) (Fig. 7d). Other mutual geochemical behaviors of Cd and Zn in the Baltic are the complex formations with bi/polysulfides and in less soluble forms in anoxic environments (Fig. 7d) (Pohl and Hennings 1999). These could explain the higher concentrations of Cd and Zn in the sediment profiles of stations 5–10 compared to those of the Gulf of Bothnia and stations 11 and 12. In the Gotland Basin (stations 5–7), studies have revealed the existence of iron sulfides, which can contain high concentrations of trace elements (Perttilä 2003). Stations 8–12, compared to the central Baltic Proper concentrations, have an increase in surface sediment concentrations and peaks that may be related to water depth, such that in deeper areas (station 8), more anoxic conditions exist and favor the formation of metal-organic complexes and sulfides and, as a result, increase the surface sediment concentrations (Borg and Jonsson 1996; Beldowski et al. 2010). Station 8 is close to municipality emission hotspots (HELCOM 2013), which might contribute to the elevated levels of Cd, Zn, and Pb. Station 9 is also very deep, which explains the anoxic conditions favoring the precipitation of metals under anoxic conditions. Stations 11 and 12 show lower surface sediment concentrations as well as lower values than those in other cores in the west Baltic Proper. This result might be explained by the frequent freshwater import from the Bornholm into the Baltic Proper (Mohrholz 2018). The local inputs of fresh waters will increase the oxygen levels of the water in the area and eliminate the reducing conditions that contribute to the precipitation of Mn (Perttilä 2003). This process will favor adsorptions of trace metals onto the surface Mn oxides. For lead, the limited transport of Pb contacting SPM and other sediments into the Baltic Proper basin might explain the restrained concentrations reported by Perttilä (2003) and this study. Lead in anoxic sediments is adsorbed to sulfides, forming a predominant and stable form (Neff 2002c). However, with excessive amounts of sulfides, Pb forms unstable complexes (Neff 2002c). In addition, Borg and Jonsson (1996) and Rogan Šmuc et al. (2018) report higher concentrations of Pb from reduced (anoxic) sediments, which is related to the formation of stable Fe and Mn complexes (Fig. 7d). However, lead is also reported to be highly unstable in anoxic sediments, with higher flux from the sediments to the water column (Fig. 7d) (Neff 2002c). This result could explain the higher concentrations of Pb in the topmost sediments of the Gulf of Bothnia and in the shallow sites (stations 11 and 12) of the Baltic Proper (Figs. 3, 4, 5, and 6).
Conclusion
This report illustrates the peak concentrations of Cd, Zn, and Pb that date back to the 1970–1980s. The reported concentrations of metals in surface sediments (0–1 cm) of the Gulf of Bothnia and Baltic Proper are higher than the calculated pre-industrial values. The calculated pre-industrial values in this study for the Baltic Proper are elevated compared to the overall pre-industrial values established by the Swedish EPA. A trend of improvement, possibly related to actions taken by the countries around the Baltic Sea, is present. Stations deviated from this trend, increasing close in the surface layer, which might be explained by surface water interactions with the sediments. Varying metal concentrations in the Baltic Sea are partly related to redox conditions promoted by climate change, limitation of oxic water mixing, and eutrophication. In addition, the presence of point contamination sources, Mn nodules, sulfides, and Mn/Fe oxides contribute to these concentrations. The higher concentrations of Cd, Zn, and Pb observed in the Baltic Proper and some part of the Gulf of Bothnia are also related to the reducing conditions of the deep waters in this area, altering metal forming complexes and resulting in precipitation. However, the precipitation and dilution of metals such as Pb will need further investigation with regard to oxic/anoxic waters. Thus, it could be concluded that anoxic sea waters, which tend to become more important with the advance of eutrophication and climate change, may contribute to higher metal concentrations in sediments.
References
Abadi M, Zamani A, Parizanganeh A, Khosravi Y, Badiee H (2019) Distribution pattern and pollution status by analysis of selected heavy metal amounts in coastal sediments from the southern Caspian Sea. Environ Monit Assess 191:144. https://doi.org/10.1007/s10661-019-7261-2
Apler A, Josefsson S (2016) Swedish status and trend monitoring programme Chemical contamination in offshore sediments 2003–2014
Aytekin T, Kargın D, Çoğun HY, Temiz Ö, Varkal HS, Kargın F (2019) Accumulation and health risk assessment of heavy metals in tissues of the shrimp and fish species from the Yumurtalik coast of Iskenderun Gulf, Turkey. Heliyon 5:e02131. https://doi.org/10.1016/j.heliyon.2019.e02131
Bastami KD, Neyestani MR, Shemirani F, Soltani F, Haghparast S, Akbari A (2015) Heavy metal pollution assessment in relation to sediment properties in the coastal sediments of the southern Caspian Sea. Mar Pollut Bull 92:237–243. https://doi.org/10.1016/j.marpolbul.2014.12.035
Beldowski J, Kulinski K, Darecki M, Beldowska M (2010) Vertical mercury, cadmium and lead distribution at two stratified stations in the Southern Baltic Sea. In: Proceedings of 15th International Conference on Heavy Metals in the Environment, (p. 1071). Department of Analytical Chemistry, Chemical Faculty, Gdansk University of Technology, Gdansk., Poland, pp 537–540
Borg H, Jonsson P (1996) Large-scale metal distribution in Baltic Sea sediments. Mar Pollut Bull 32:8–21. https://doi.org/10.1016/0025-326X(95)00103-T
Carstensen J, Andersen JH, Gustafsson BG, Conley DJ (2014a) Deoxygenation of the Baltic Sea during the last century. Proc Natl Acad Sci 111:5628–5633. https://doi.org/10.1073/pnas.1323156111
Carstensen J, Conley DJ, Bonsdorff E, Gustafsson BG, Hietanen S, Janas U, Jilbert T, Maximov A, Norkko A, Norkko J, Reed DC, Slomp CP, Timmermann K, Voss M (2014b) Hypoxia in the Baltic Sea: biogeochemical cycles, benthic fauna, and management. AMBIO 43:26–36. https://doi.org/10.1007/s13280-013-0474-7
Ciubotariu D, Ghiciuc CM, Lupușoru CE (2015) Zinc involvement in opioid addiction and analgesia—should zinc supplementation be recommended for opioid-treated persons? Subst Abuse Treat Prev Policy 10. https://doi.org/10.1186/s13011-015-0025-2
Conley DJ, Humborg C, Rahm L, Savchuk OP, Wulff F (2002) Hypoxia in the Baltic Sea and basin-scale changes in phosphorus biogeochemistry. Environ Sci Technol 36:5315–5320
Daoji L, Daler D (2004) Ocean pollution from land-based sources: East China Sea, China. Ambio 33:107–113
de Paula Filho FJ, Marins RV, de Lacerda LD, Aguiar JE, Peres TF (2015) Background values for evaluation of heavy metal contamination in sediments in the Parnaíba River Delta estuary, NE/Brazil. Mar Pollut Bull 91:424–428. https://doi.org/10.1016/j.marpolbul.2014.08.022
Fukue M, Yanai M, Sato Y, Fujikawa T, Furukawa Y, Tani S (2006) Background values for evaluation of heavy metal contamination in sediments. J Hazard Mater 136:111–119. https://doi.org/10.1016/j.jhazmat.2005.11.020
Gałuszka A, Migaszewski Z (2011) Geochemical background—an environmental perspective. Mineralogia 42:7–17. https://doi.org/10.2478/v10002-011-0002-y
Gill R (2014) Modern analytical geochemistry: an introduction to quantitative chemical analysis techniques for Earth, environmental and materials scientists. Routledge
Håkansson B, Alenius P, Brydsten L (1996) Physical environment in the Gulf of Bothnia. Ambio:5–12
HELCOM (2013) Implementation of the Hot Spots programme, 1992-2013. Helsinki Commission
HELCOM (2018) HELCOM Assessment on maritime activities in the Baltic Sea 2018 (No. 152), Baltic Sea Environment Proceedings. Helsinki Commission, Helsinki, Finland. 256
Ingri J, Widerlund A, Suteerasak T, Bauer S, Elming S-Å (2014) Changes in trace metal sedimentation during freshening of a coastal basin. Mar Chem 167:2–12. https://doi.org/10.1016/j.marchem.2014.06.010
Jönsson A, Danielsson Å, Rahm L (2005) Bottom type distribution based on wave friction velocity in the Baltic Sea. Cont Shelf Res 25:419–435
Josefsson S, Apler A (2019) Miljöföroreningar i utsjösediment - geografiska mönster och tidstrender
Kabel K, Moros M, Porsche C, Neumann T, Adolphi F, Andersen TJ, Siegel H, Gerth M, Leipe T, Jansen E, Sinninghe Damsté JS (2012) Impact of climate change on the Baltic Sea ecosystem over the past 1,000 years. Nat Clim Chang 2:871–874. https://doi.org/10.1038/nclimate1595
Leivuori M, Niemistö L (1995) Sedimentation of trace metals in the Gulf of Bothnia. Chemosphere 31:3839–3856. https://doi.org/10.1016/0045-6535(95)00257-9
Mohrholz V (2018) Major Baltic Inflow Statistics—revised. Front Mar Sci 5. https://doi.org/10.3389/fmars.2018.00384
Neff JM (2002a) Chapter 5—cadmium in the ocean. In: Bioaccumulation in marine organisms. Elsevier, Oxford, pp 89–102
Neff JM (2002b) Chapter 10—zinc in the ocean. In: Bioaccumulation in marine organisms. Elsevier, Oxford, pp 175–189
Neff JM (2002c) Chapter 9—lead in the ocean. In: Bioaccumulation in marine organisms: effect of contaminants from oil well produced water. Elsevier, pp 161–174
Niemistö L (1974) A gravity corer for studies of soft sediments. 238:33–38
Östlund P, Sternbeck J (2001) Total lead and stable lead isotopes in Stockholm sediments. Water Air Soil Pollut Focus 1:229–239. https://doi.org/10.1023/A:1017568507985
Öztürk M (1995) Trends of trace metal (Mn, Fe, Co, Ni, Cu, Zn, Cd and Pb) distributions at the oxic-anoxic interface and in sulfidic water of the Drammensfjord. Mar Chem 48:329–342
Perttilä M (2003) Contaminants in the Baltic Sea sediments: results of the 1993 ICES/HELCOM Sediment Baseline Study. Finnish Institute of Marine Research
Pohl C, Hennings U (1999) The effect of redox processes on the partitioning of Cd, Pb, Cu, and Mn between dissolved and particulate phases in the Baltic Sea. Mar Chem 65:41–53. https://doi.org/10.1016/S0304-4203(99)00009-2
Radzevièius R (2002) Main trends in accumulation of trace elements from surface sediments of the Baltic Sea (Lithuanian waters). Baltica 15:63–73
Reimann C, Garrett RG (2005) Geochemical background—concept and reality. Sci Total Environ 350:12–27. https://doi.org/10.1016/j.scitotenv.2005.01.047
Remeikaitė-Nikienė N, Garnaga-Budrė G, Lujanienė G, Jokšas K, Stankevičius A, Malejevas V, Barisevičiūtė R (2018) Distribution of metals and extent of contamination in sediments from the south-eastern Baltic Sea (Lithuanian zone). Oceanologia 60:193–206. https://doi.org/10.1016/j.oceano.2017.11.001
Rodrigues SM, Glegg GA, Pereira ME, Duarte AC (2009) Pollution problems in the Northeast Atlantic: lessons learned for emerging pollutants such as the platinum group elements. Ambio 38:17–23
Rogan Šmuc N, Dolenec M, Kramar S, Mladenović A (2018) Heavy metal signature and environmental assessment of nearshore sediments: port of Koper (Northern Adriatic Sea). Geosciences 8:398. https://doi.org/10.3390/geosciences8110398
Rtudio Team (2018) RStudio. RStudio, Inc, Boston
Skerfving S, Bencko V, Vahter M, Schütz A, Gerhardsson L (1999) Environmental health in the Baltic region—toxic metals. Scand J Work Environ Health 25:40–64
Suess E, Erlenkeuser H (1975) Geochemistry, carbon isotope ratio and radiocarbon ages of sediment core GIK1093-2 from Bornholm Basin, Baltic Sea. Supplement to: Suess, E; Erlenkeuser, H (1975): History of metal pollution and carbon input in Baltic Sea sediments. Meyniana, 27, 63-75, https://doi.org/10.2312/meyniana.1975.27.63
Swedish EPA (2000) Environmental quality criteria—coasts and seas. Swedish Environmental Protection Agency, Report 5052, ISBN 91-620-5052-4
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metals toxicity and the environment. EXS 101:133–164. https://doi.org/10.1007/978-3-7643-8340-4_6
Tornero V, Hanke G (2016) Chemical contaminants entering the marine environment from sea-based sources: a review with a focus on European seas. Mar Pollut Bull 112:17–38. https://doi.org/10.1016/j.marpolbul.2016.06.091
Vallius H (2014) Heavy metal concentrations in sediment cores from the northern Baltic Sea: declines over the last two decades. Mar Pollut Bull 79:359–364
Zaborska A (2014) Anthropogenic lead concentrations and sources in Baltic Sea sediments based on lead isotopic composition. Mar Pollut Bull 85:99–113. https://doi.org/10.1016/j.marpolbul.2014.06.013
Zgłobicki W, Lata L, Plak A, Reszka M (2011) Geochemical and statistical approach to evaluate background concentrations of Cd, Cu, Pb and Zn (case study: Eastern Poland). Environ Earth Sci 62:347–355. https://doi.org/10.1007/s12665-010-0529-z
Acknowledgments
We thank the Swedish Geological Survey (SGU – Sveriges geologiska undersökning) for the research grant no. 36-2033/2018, and Linnaeus University for a PhD scholarship to Sina Shahabi-Ghahfarokhi. We also thank the Swedish EPA which allowed the use of sampled sediment material.
Funding
Open access funding provided by Linnaeus University. This study was supported by research grant no. 36-2033/2018 (SGU–Sveriges geologiska undersökning) which was used to analyze the total metal concentrations of the samples and PhD scholarship of Linnaeus University to Sina Shahabi-Ghahfarokhi, corresponding author, which allowed this research to be conducted.
Author information
Authors and Affiliations
Contributions
SSHGH post-treated the samples, defined the methodology, interpreted the data, and was a major contributor in writing the paper. SSHGH also performed the Visualization of the figures included in the current manuscript. MA helped to interpret the data and give input for the structure of the paper as well scientific advice. AA and SJ helped to gather the samples which was shared with the corresponding author; in addition, they also contributed to the input of the main text. KK has given scientific input and has checked the text for each revision. MK, as main supervisor, was the main fund raiser and helped with the interpretation and input of the main text, in addition. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Availability of data and materials
All data samples analyzed during this study that are included in this published article can be found in the attached Appendices file.
Additional information
Responsible editor: Vedula VSS Sarma
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shahabi-Ghahfarokhi, S., Josefsson, S., Apler, A. et al. Baltic Sea sediments record anthropogenic loads of Cd, Pb, and Zn. Environ Sci Pollut Res 28, 6162–6175 (2021). https://doi.org/10.1007/s11356-020-10735-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10735-x