Abstract
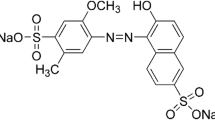
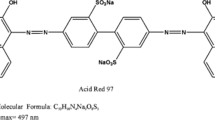
The wastes from the macro-fungus Agaricus bisporus were used as an eco-friendly and low-cost adsorbent for the treatment of colored effluents containing the recalcitrant dyes, acid red 97 (AR97) and crystal violet (CV). The macro-fungal waste presented an amorphous structure, composed of particles with different sizes and shapes. Also, it presents typical functional chemical groups of proteins and carbohydrates with a point of zero charge of 4.6. The optimum conditions for the dosage were found to be as follows: 0.5 g L−1 with an initial pH at 2.0 for the AR97 and 8.0 for the CV. From the kinetic test, it was found that it took 210 min and an adsorption capacity of 165 mg g−1 for the AR97. Concerning the CV kinetics, it took 120 min to reach the equilibrium and it achieved an adsorption capacity of 165.9 mg g−1. The Elovich model was the most proper model for describing the experimental data, achieving an R2 ≥ 0.997 and MSE ≤ 36.98 (mg g−1)2. The isotherm curves were best represented by the Langmuir model, predicting maximum adsorption capacity of 372.69 and 228.74 mg g−1 for the AR97 and CV, respectively. The process was spontaneous and favorable for both dyes. The ∆H0 values were 9.53 and 10.69 kJ mol−1 for AR97 and CV, respectively, indicating physical and endothermic adsorption. Overall, the wastes from Agaricus bisporus have the potential to adsorb cationic and anionic dyes, thus solving environmental problems related to water quality and residue disposal.









Similar content being viewed by others
References
Akar ST, Gorgulu A, Kaynak Z, Anilan B, Akar T (2009) Biosorption of reactive blue 49 dye under batch and continuous mode using a mixed biosorbent of macro-fungus Agaricus bisporus and Thuja orientalis cones. Chem Eng J 148:26–34
Bairagi H, Khan MMR, Ray L, Guha AK (2011) Adsorption profile of lead on Aspergillus versicolor: a mechanistic probing. J Hazard Mater 186:756–764
Bankole PO, Adekunle AA, Govindwar SP (2018) Enhanced decolorization and biodegradation of acid red 88 dye by newly isolated fungus, Achaetomium strumarium. J Environ Chem Eng 6:1589–1600
Bayramoğlu G, Yakup Arıca M (2007) Biosorption of benzidine based textile dyes “direct blue 1 and direct red 128” using native and heat-treated biomass of Trametes versicolor. J Hazard Mater 143:135–143
Bellatin L, Herrera O, Navarro A, Sun-kou R, Llanos B (2014) Biosorption study of acid red 18, basic blue 99 and basic yellow 57, present in hair dyes, with residues of green tea leaves. Rev Soc Chem Peru 80
Chang R, Thoman JW (2014) Physical chemistry for the chemical sciences. University Science Books
Demarchi CA, Campos M, Rodrigues CA (2013) Adsorption of textile dye reactive red 120 by the chitosan-Fe(III)-crosslinked: batch and fixed-bed studies. J Environ Chem Eng 1:1350–1358
Djelad A, Mokhtar A, Khelifa A, Bengueddach A, Sassi M (2019) Alginate-whey an effective and green adsorbent for crystal violet removal: kinetic, thermodynamic and mechanism studies. Int J Biol Macromol 139:944–954
Dotto GL, McKay G (2020) Current scenario and challenges in adsorption for water treatment. J Environ Chem Eng 8:103988
Duarte AL, DaBoit K, Oliveira MLS, Teixeira EC, Schneider IL, Silva LFO (2019) Hazardous elements and amorphous nanoparticles in historical estuary coal mining area. Geosci Front 10:927–939
El-Bindary AA, Diab MA, Hussien MA, El-Sonbati AZ, Eessa AM (2014) Adsorption of acid red 57 from aqueous solutions onto polyacrylonitrile/activated carbon composite. Spectrochim Acta Part A: Mol Biomol Spect 124:70–77
Elovich SY, Larinov OG (1962) Theory of adsorption from solutions of non-electrolytes on solid (I) equation adsorption from solutions and the analysis of its simplest form, (II) verification of the equation of adsorption isotherm from solutions. Zvestiya Akademii Nauk SSSR 2:209–216
Franco DSP, Georgin J, Drumm FC, Netto MS, Allasia D, Oliveira MLS, Dotto GL (2020) Araticum (Annona crassiflora) seed powder (ASP) for the treatment of colored effluents by biosorption. Environ Sci Pollut Res 27:11184–11194
Freundlich H (1906) Ubber die adsorption in lusungen. J Am Chem Soc 61:2–28
Fu Y, Viraraghavan T (2002) Dye biosorption sites in Aspergillus niger. Bioresour Technol 82:139–145
Georgin J, Alves E, Drumm F, Tonato D, Grassi P, Piccin JS, Oliveira MLS, Dotto GL, Mazutti M (2019b) Application of Beauveria bassiana spore waste as adsorbent to uptake acid red 97 dye from aqueous medium. Environ Sci Pollut Res 26:36967–36977
Georgin J, Drumm FC, Grassi P, Franco D, Allasia D, Dotto GL (2018a) Potential of Araucaria Angustifolia bark as an adsorbent to remove gentian violet dye from aqueous effluents. Water Sci Technol 78:1693–1703
Georgin J, Franco DSP, Grassi P, Tonato D, Piccilli DGA, Meili L, Dotto GL (2019a) Potential of Cedrella fissilis bark as an adsorbent for the removal of red 97 dye from aqueous effluents. Environ Sci Pollut Res 26:19207–19219
Georgin J, Marques BS, Peres EC, Allasia D, Dotto GL (2018b) Biosorption of cationic dyes by Pará chestnut husk (Bertholletia excelsa). Water Sci Technol 77:1612–1621
Ghazali A, Shirani M, Semnani A, Zare-Shahabadi V, Nekoeinia M (2018) Optimization of crystal violet adsorption onto. Date palm leaves as a potent biosorbent from aqueous solutions using response surface methodology and ant colony. J Environ Chem Eng 6:3942–3950
Giles CH, Smith D, Huitson A (1974) A general treatment and classification of the solute adsorption isotherm. I. Theoretical. J Colloid Interface Sci 47:755–765
Gopi S, Pius A, Thomas S (2016) Enhanced adsorption of crystal violet by synthesized and characterized chitin nanowhiskers from shrimp shell. J water Process Eng 14:1–8
Graba Z, Hamoudi S, Bekka D, Bezzi N, Boukherroub R (2015) Influence of adsorption parameters of basic red dye 46 by the rough and treated Algerian natural phosphates. J Ind Eng Chem 25:229–238
Grassi P, Reis C, Drumm FC, Georgin J, Tonato D, Escudero LB, Dotto GL (2019) Biosorption of crystal violet dye using inactive biomass of the fungus Diaporthe schini. Water Sci Technol 79:709–717
Gupta VK, Pathania D, Sharma S, Agarwal S, Singh P (2013) Remediation and recovery of methyl orange from aqueous solution onto acrylic acid grafted Ficus carica fiber: isotherms, kinetics, and thermodynamics. J Mol Liq 177:325–334
Gupta VK, Ali I, Saleh TA, Siddiqui MN, Agarwal S (2012) Chromium removal from water by activated carbon developed from waste rubber tires. Environ Sci Pollut Res 20:1261–1268
Gupta VK, Rastogi A, Dwivedi MK, Mohan D (1997) Process development for the removal of zinc and cadmium from wastewater using slag-A blast furnace waste material. Sep Sci Technol 32:2883–2912
Güzel F, Sayğılı H, Akkaya Sayğılı G, Koyuncu F (2015) New low-cost nanoporous carbonaceous adsorbent developed from carob (Ceratonia siliqua) processing industry waste for the adsorption of anionic textile dye: characterization, equilibrium and kinetic modeling. J Mol Liq 206:244–255
Heibati B, Rodriguez-Couto S, Al-Ghouti MA, Asif M, Tyagi I, Agarwal S, Gupta VK (2015) Kinetics and thermodynamics of enhanced adsorption of the dye AR 18 using activated carbons prepared from walnut and poplar woods. J Mol Liq 208:99–105
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Khodam F, Rezvani Z, Amani-Ghadim AR (2015) Enhanced adsorption of acid red 14 by co-assembled LDH/MWCNTs nanohybrid: optimization, kinetic and isotherm. J Ind Eng Chem 21:1286–1294
Kumar R, Ahmad R (2011) Biosorption of hazardous crystal violet dye from aqueous solution onto treated ginger waste (TGW). Desalination 265:112–118
Lagergren S (1898) Zur Theorie der Sogenannten Adsorption Gelöster Stoffe. Kung Svenska Vetenskap 24:1–39
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica, and platinum. J Amer Chem Soc 40:1361–1403
Li X, Zhang D, Sheng F, Qing H (2018) Adsorption characteristics of copper, zin and mercury by four kinds of immobilized fungi residues. Ecotoxicol Environ Saf 147:357–366
Liang YD, He YJ, Wang TT, Lei LH (2019) Adsorptive removal of gentian violet from aqueous solution using CoFe2O4/activated carbon magnetic composite. J Water Proc Eng 27:77–88
Lima EC, Hosseini-Bandegharaei A, Moreno-Piraján JC, Anastopoulos I (2018) A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the Van’t Hoof equation for calculation of thermodynamic parameters of adsorption. J Mol Liq 273:425–434
Liu Y, Shen L (2008) A general rate law equation for biosorption. Biochem Eng J 38:390–394
Maurya NS, Mittal AK, Cornel P, Rother E (2006) Biosorption of dyes using dead macro fungi: effect of dye structure, ionic strength, and pH. Bioresour Technol 97:512–521
Mittal A, Mittal J, Malviya A, Gupta VK (2010) Removal and recovery of Chrysoidine Y from aqueous solutions by waste materials. J Colloid Interface Sci 344:497–507
Miyah Y, Lahrichi A, Idrissi M, Boujraf S, Taouda H, Zerrouq F (2017) Assessment of adsorption kinetics for removal potential of crystal violet dye from aqueous solutions using Moroccan pyrophyllite. J Assoc Arab Univ Basic Appl Sci 23:20–28
Mohammadi N, Khani H, Gupta VK, Amereh E, Agarwal S (2011) Adsorption process of methyl orange dye onto mesoporous carbon material-kinetic and thermodynamic studies. J Colloid Interface Sci 362:457–462
Nekouei F, Nekouei S, Tyagi I, Gupta VK (2015) Kinetic, thermodynamic and isotherm studies for acid blue 129 removal from liquids using copper oxide nanoparticle-modified activated carbon as a novel adsorbent. J Mol Liq 201:124–133
Oliveira M, Izquierdo M, Querol X, Lieberman RN, Saikia BK, Silva LFO (2019) Nanoparticles from construction wastes: a problem to health and the environment. J Clean Prod 219:236–243
Pal A, Pan S, Saha S (2013) Synergistically improved adsorption of anionic surfactant and crystal violet on chitosan hydrogel beads. Chem Eng J 217:426–434
Pathania D, Katwal R, Kaur H (2016) Enhanced photocatalytic activity of electrochemically synthesized aluminum oxide nanoparticles. Int J Miner Metall Mater 23:358–371
Pavan FA, Lima EC, Dias SLP, Mazzocato AC (2008) Methylene blue biosorption from aqueous solutions by yellow passion fruit waste. J Hazard Mater 150:703–712
Postai DL, Demarchi CA, Zanatta F, Melo DCC, Rodrigues CA (2016) Adsorption of rhodamine B and methylene blue dyes using the waste of seeds of Aleurites Moluccana, a low-cost adsorbent. Alexandria Eng J 55:1713–1723
Rigueto CVT, Piccin JS, Dettmer A, Rosseto M, Dotto GL, Schimitz APO, Perondi D, Freitas TSM, Loss RA, Geraldi CAQ (2020) Water hyacinth (Eichhornia crassipes) roots, an amazon natural waste, as an alternative biosorbent to uptake a reactive textile dye from aqueous solutions. Ecol Eng 150:105817
Rojas JC, Sánchez NE, Schneider I, Oliveira MLS, Teixeira EC, Silva LFO (2019) Exposure to nanometric pollutants in primary schools: environmental implications. Urban Clim 27:412–419
Román S, Valente Nabais JM, Ledesma B, González JF, Laginhas C, Titirici MM (2013) Production of low-cost adsorbents with tunable surface chemistry by the conjunction of hydrothermal carbonization and activation processes. Microporous Mesoporous Mater 165:127–133
Sarma GK, Sen Gupta S, Bhattacharyya KG (2016) Adsorption of crystal violet on raw and acid-treated montmorillonite, K10 in aqueous suspension. J Environ Manag 171:1–10
Shakoor S, Nasar A (2018) Adsorptive decontamination of synthetic wastewater containing crystal violet dye by employing Terminalia arjuna sawdust waste. Ground Sustain Dev 7:30–38
Sharma G, Kumar A, Naushad M, García-Peñas A, Al-Muhtaseb AH, Ghfar AA, Stadler FJ (2018) Fabrication and characterization of gum arabic-cl-poly (acrylamide) nano hydrogel for effective adsorption of crystal violet dye. Carbohydr Polym 202:444–453
Sonai GG, de Souza SMAGU, de Oliveira D, de Souza AAU (2016) The application of textile sludge adsorbents for the removal of reactive red 2 dye. J Environ Manag 168:149–156
Srikantan C, Suraishkumar GK, Srivastava S (2018) Effect of light on the kinetics and equilibrium of the textile dye (reactive red 120) adsorption by Helianthus annuus hairy roots. Bioresour Technol 257:84–91
Streit AFM, Côrtes LN, Druzian SP, Godinho M, Collazzo GC, Perondi D, Dotto GL (2019) Development of high quality activated carbon from biological sludge and its application for dyes removal from aqueous solutions. Sci Total Environ 660:277–287
Tahir N, Bhatti HN, Iqbal M, Noreen S (2017) Biopolymers composites with peanut hull waste biomass and application for crystal violet adsorption. Int J Biol Macromol 94:210–220
Thue PS, Sophia AC, Lima EC, Wamba AGN, de Alencar WS, dos Reis GS, Dias SLP (2018) Synthesis and characterization of a novel organic-inorganic hybrid clay adsorbent for the removal of acid red 1 and acid green 25 from aqueous solutions. J Clean Prod 171:30–44
Vetter J (2007) Chitin content of cultivated mushrooms Agaricus bisporus, Pleurotus ostreatus, and Lentinula edodes. Food Chem 102:6–9
Wu FC, Tseng RL, Juang RS (2009) Characteristics of the Elovich equation used for the analysis of adsorption kinetics in dye-chitosan systems. Chem Eng J 150:366–373
Xu D, Gu C, Chen X (2013) Adsorption and removal of acid red 3R from aqueous solution using flocculent humic acid isolated from lignite. Procedia Environ Sci 18:127–134
Zazycki MA, Godinho M, Perondi D, Foletto EL, Collazzo GC, Dotto GL (2018) New biochar from pecan nutshells as an alternative adsorbent for removing reactive red 141 from aqueous solutions. J Clean Prod 171:57–65
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial Responsibility: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 22 kb)
Rights and permissions
About this article
Cite this article
Drumm, F.C., Franco, D.S.P., Georgin, J. et al. Macro-fungal (Agaricus bisporus) wastes as an adsorbent in the removal of the acid red 97 and crystal violet dyes from ideal colored effluents. Environ Sci Pollut Res 28, 405–415 (2021). https://doi.org/10.1007/s11356-020-10521-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10521-9