Abstract
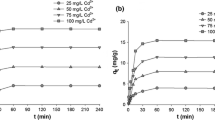
In this study, a new adsorbent of silicon-doped magnesium oxide (SMG) was developed for the recovery of nutrients from wastewater. The adsorption conditions including adsorbent dosage, initial solution pH, contact time, coexisting substances, N/P molar ratios, and reaction temperature were investigated. Analysis of field emission scanning electron microscopy-energy dispersive spectrometer (FESEM-DES) and specific surface areas (BET) showed that SMG was a mesoporous adsorbent with SBET of 108.31 m2/g. The recycled sediment (RS) was identified as almost pure struvite via X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), and X-ray photoelectron spectroscopy (XPS). The recovery efficiencies of SMG reached 43.25% of ammonia nitrogen and 97.31% of phosphate at dosage of 0.3 g/L, initial solution pH of 7.0, contact time of 20 min, and temperature of 298 K. Under the optimal reaction conditions, the maximum adsorption capacities of SMG were 170.93 mg/g of ammonia nitrogen and 420.89 mg/g of phosphate at N/P molar ratio of 1.5:1. Coexisting humic acid (HA), calcium (Ca2+), acetic acid (AA), and ferric ions (Fe3+) in nutrient solution hindered the struvite ordered precipitation. The adsorption process followed pseudo-second-order and Elovich kinetic models and was well described by both the Langmuir and Freundlich isotherms at room temperature. All results indicated that the most likely mechanism of nutrients recovery from wastewater was chemical precipitation and proved that SMG was a high-efficiency adsorption material in a wide pH range of 3.0–9.0 for simultaneous recovery of nutrients from wastewater.






Similar content being viewed by others
References
Acelas NY, Martin BD, Lopez D, Jefferson B (2015) Selective removal of phosphate from wastewater using hydrated metal oxides dispersed within anionic exchange media. Chemosphere 119:1353–1360
Aguado J, Arsuaga JM, Arencibia A (2005) Adsorption of aqueous mercury (II) on propylthiol-functionalized mesoporous silica obtained by cocondensation. Ind Eng Chem Res 44(10):3665–3671
APHA, AWWA, WPFC (2012) Standard methods for the examination of water and wastewater. American Public Health Association, Washington, DC
Azizian S (2004) Kinetic models of sorption: a theoretical analysis. J Colloid Interface Sci 276(1):47–52
Barbosa SG, Peixoto L, Meulman B, Alves MM, Pereira MA (2016) A design of experiments to assess phosphorous removal and crystal properties in struvite precipitation of source separated urine using different Mg sources. Chem Eng J 298:146–153
Bibby A, Mercier L (2002) Mercury (II) ion adsorption behavior in thiol-functionalized mesoporous silica microspheres. Chem Mater 14(4):1591–1597
Blazquez G, Martin-Lara MA, Dionisio-Ruiz E, Tenorio G, Calero M (2011) Evaluation and comparison of the biosorption process of copper ions onto olive stone and pine bark. J Ind Eng Chem 17(5–6):824–833
Bouropoulos NC, Koutsoukos PG (2000) Spontaneous precipitation of struvite from aqueous solutions. J Cryst Growth 213(3–4):381–388
Capdevielle A, Sykorova E, Beline F, Daumer ML (2014) Kinetics of struvite precipitation in synthetic biologically treated swine wastewaters. Environ Technol 35(10):1250–1262
Chen Y, Tang J, Li W, Zhong Z, Yin J (2015) Thermal decomposition of magnesium ammonium phosphate and adsorption properties of its pyrolysis products toward ammonia nitrogen. Trans Nonferrous Met Soc 25(2):497–503
Chen L, Liu F, Wu Y, Zhao L, Li Y, Zhang X, Qian J (2018) In situ formation of La (OH)3-poly (vinylidene fluoride) composite filtration membrane with superior phosphate removal properties. Chem Eng J 347:695–702
Crini G, Badot PM (2008) Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: a review of recent literature. Prog Polym Sci 33(4):399–447
Frost RL, Weier ML, Erickson KL (2004) Thermal decomposition of struvite- implications for the decomposition of kidney stones. J Therm Anal Calorim 76(3):1025–1033
Guaya D, Valderrama C, Farran A, Armijos C, Cortina JL (2015) Simultaneous phosphate and ammonium removal from aqueous solution by a hydrated aluminum oxide modified natural zeolite. Chem Eng J 271:204–213
Haddad K, Jellali S, Jeguirim M, Trabelsi AB, Limousy L (2018) Investigations on phosphorus recovery from aqueous solutions by biochars derived from magnesium-pretreated cypress sawdust. J Environ Manag 216:305–314
Hao H, Wang Y, Shi B (2019) NaLa(CO3)2 hybridized with Fe3O4 for efficient phosphate removal: synthesis and adsorption mechanistic study. Water Res 155:1–11
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34(5):451–465
Johansson S, Ruscalleda M, Colprim J (2017) Phosphorus recovery through biologically induced precipitation by partial nitritation-anammox granular biomass. Chem Eng J 327:881–888
Karadag D, Koroglu OE, Ozkaya B, Cakmakci M (2015) A review on anaerobic biofilm reactors for the treatment of dairy industry wastewater. Process Biochem 50(2):262–271
Karageorgiou K, Paschalis M, Anastassakis GN (2007) Removal of phosphate species from solution by adsorption onto calcite used as natural adsorbent. J Hazard Mater 139(3):447–452
Lee WPC, Mah SK, Leo CP, Wu T, Chai SP (2014) Performance studies of phosphorus removal using cross-flow nanofiltration. Desalin Water Treat 52(31–33):5974–5982
Luo Z, Peng J, Wang D, Yang J (2019) Recovery of phosphate from piggery biogas slurry by ultrasonication, aeration and addition of MgO desulfurization waste residue. J Clean Prod 211:865–873
Pastor L, Mangin D, Barat R, Seco A (2008) A pilot-scale study of struvite precipitation in a stirred tank reactor: conditions influencing the process. Bioresour Technol 99(14):6285–6291
Quintana M, Colmenarejo MF, Barrera J, Garcia G, Garcia E, Bustos A (2004) Use of a byproduct of magnesium oxide production to precipitate phosphorus and nitrogen as struvite from wastewater treatment liquors. J Agric Food Chem 52:294–299
Scholz RW, Ulrich AE, Eilitta M, Roy A (2013) Sustainable use of phosphorus: a finite resource. Sci Total Environ 461:799–803
Shih YJ, Abarca RRM, de Luna MDG, Huang Y, Lu M (2017) Recovery of phosphorus from synthetic wastewaters by struvite crystallization in a fluidized-bed reactor: effects of pH, phosphate concentration and coexisting ions. Chemosphere 173:466–473
Si Q, Zhu Q, Xing Z (2018) Simultaneous removal of nitrogen and phosphorus by magnesium-modified calcium silicate core-shell material in water. Ecotox Environ Safe 163:656–664
Song Y, Shan D, Chen R, Zhang F, Han E (2009) A novel phosphate conversion film on Mg-8.8Li alloy. Surf. Coat. Tech. 203(9):1107–1113
Tansel B, Lunn G, Monje O (2018) Struvite formation and decomposition characteristics for ammonia and phosphorus recovery: a review of magnesium-ammonia-phosphate interactions. Chemosphere 194:504–514
Van der Houwen JAM, Valsami-Jones E (2001) The application of calcium phosphate precipitation chemistry to phosphorus recovery: the influence of organic ligands. Environ Technol 22(11):1325–1335
Wang H, Wang X, Xia P, Song J, Ma R, Jing H, Zhang Z, Cheng X, Zhao J (2017) Eco-friendly synthesis of self-existed magnesium oxide supported nanorod-like palygorskite for enhanced and simultaneous recovery of nutrients from simulated wastewater through adsorption and in-situ struvite formation. Appl Clay Sci 135:418–426
Wang J, Ye X, Zhang Z, Ye Z, Chen S (2018a) Selection of cost-effective magnesium sources for fluidized struvite crystallization. J Environ Sci-China 70:144–153
Wang Q, Li J, Tang P, Fang L, Poon CS (2018b) Sustainable reclamation of phosphorus from incinerated sewage sludge ash as value-added struvite by chemical extraction, purification and crystallization. J Clean Prod 181:717–725
Warmadewanthi B, Liu J (2009) Selective precipitation of phosphate from semiconductor wastewater. J Environ Eng 135(10):1063–1070
Wilfert P, Kumar PS, Korving L, Witkamp GJ, van Loosdrecht MCM (2015) The relevance of phosphorus and iron chemistry to the recovery of phosphorus from wastewater: a review. Environ Sci Technol 49(16):9400–9414
Wilfert P, Mandalidis A, Dugulan AI, Goubitz K, Korving L, Temmink H, Witkamp GJ, Van Loosdrecht MCM (2016) Vivianite as an important iron phosphate precipitate in sewage treatment plants. Water Res 104:449–460
Xia P, Wang X, Wang X, Song J, Wang H, Zhang J, Zhao J (2016) Struvite crystallization combined adsorption of phosphate and ammonium from aqueous solutions by mesoporous MgO-loaded diatomite. Colloid Surface A 506:220–227
Xie F, Wu F, Liu G, Mu Y, Feng C, Wang H, Giesy JP (2014) Removal of phosphate from eutrophic lakes through adsorption by in situ formation of magnesium hydroxide from diatomite. Environ Sci Technol 48(1):582–590
Xu H, He P, Gu W, Wang G, Shao L (2012) Recovery of phosphorus as struvite from sewage sludge ash. J Environ Sci 24(8):1533–1538
Xu K, Lin F, Dou X, Zheng M, Tan W, Wang C (2018) Recovery of ammonium and phosphate from urine as value-added fertilizer using wood waste biochar loaded with magnesium oxides. J Clean Prod 187:205–214
Yetilmezsoy K, Sapci-Zengin Z (2009) Recovery of ammonium nitrogen from the effluent of UASB treating poultry manure wastewater by MAP precipitation as a slow release fertilizer. J Hazard Mater 166(1):260–269
Yin H, Kong M (2014) Simultaneous removal of ammonium and phosphate from eutrophic waters using natural calcium-rich attapulgite-based versatile adsorbent. Desalination 351:128–137
You X, Valderrama C, Cortina JL (2017) Simultaneous recovery of ammonium and phosphate from simulated treated wastewater effluents by activated calcium and magnesium zeolites. J Chem Technol Biotechnol 92(9):2400–2409
Zeng F, Zhao Q, Jin W, Liu Y, Wang K, Lee DJ (2018) Struvite precipitation from anaerobic sludge supernatant and mixed fresh/stale human urine. Chem Eng J 344:254–261
Zhang Q, Zhao S, Ye X, Xiao W (2016) Effects of organic substances on struvite crystallization and recovery. Desalin Water Treat 57(23):10924–10933
Zhang Z, Wang X, Wang H, Zhao J (2018) Removal of Pb (II) from aqueous solution using hydroxyapatite/ calcium silicate hydrate (HAP/C-S-H) composite adsorbent prepared by a phosphate recovery process. Chem Eng J 344:53–61
Zheng Y, Wang B, Wester AE, Chen J, He F, Chen H, Gao B (2019) Reclaiming phosphorus from secondary treated municipal wastewater with engineered biochar. Chem Eng J 362:460–468
Funding
This work was supported by the National Key Research and Development Programme of China (grant no. 2016YFC0401103), the Natural Science Foundation of China (grant no. 51578016), and the Natural Science Foundation of Beijing (grant no. 8172014).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, S., Zeng, W., Xu, H. et al. Performance investigation of struvite high-efficiency precipitation from wastewater using silicon-doped magnesium oxide. Environ Sci Pollut Res 27, 15463–15474 (2020). https://doi.org/10.1007/s11356-019-07589-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-07589-3