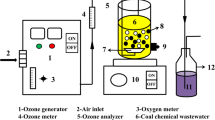
Abstract
In this work, activated carbon-supported copper(II) oxide (CuO/AC) was prepared and used to degrade heavy oil refinery wastewater (HORW) by catalytic ozonation with the aim to develop low-cost and high-efficient supported-catalysts for degrading real recalcitrant industrial wastewater. Supported-catalyst CuO/AC was characterized by X-ray diffraction (XRD), N2-physisorption, scanning electronic microscope (SEM), transmission electron microscope (TEM), and X-ray fluorescence (XRF). The degradation was mainly evaluated by chemical oxygen demand (COD), total organic carbon (TOC), 5-day biochemical oxygen demand (BOD5), biodegradability and toxicity. Compared with unsupported-catalyst CuO or the mixed system of activated-AC and unsupported-catalyst CuO, supported-catalyst CuO/AC with reduced cost exhibited significantly enhanced activity for degrading HORW (5.0 g CuO-5%/AC, 90 mg/L O3, and 7.3 pH). TEM analysis showed that the high activity of supported-catalyst CuO-5%/AC might be ascribed to the fact that CuO particles were small and highly dispersed on AC. Mass spectrum spectrometry (MS) analysis revealed that the organic components in HORW were first degraded to small molecule oxidation products, which were then oxidized or mineralized further. The influence of CuO loading, CuO/AC dose, ozone dose and initial pH on the degradation efficiency was also investigated. The results of the present work showed that CuO/AC could be a promising supported-catalyst for catalyzing ozonation degradation of HORW.









Similar content being viewed by others
References
Benitez J (2002) Principles and modern applications of mass transfer operations. Wiley, New York, pp 125–134
Cai Y (2011) Project design for treatment of super heavy oil refinery wastewater by biological process. Modern Chem Ind 31(1- supplement):366–369
Chen C, Chen H, Guo X et al (2014a) Advanced ozone treatment of heavy oil refining wastewater by activated carbon supported iron oxide. J Ind Eng Chem 20:2782–2791
Chen C, Li Y, Ma W et al (2017) Mn-Fe-Mg-Ce loaded Al2O3 catalyzed ozonation for mineralization of refractory organic chemicals in petroleum refinery wastewater. Sep Purif Technol 183:1–10
Chen C, Wei L, Guo X et al (2014b) Investigation of heavy oil refinery wastewater treatment by integrated ozone and activated carbon-supported manganese oxides. Fuel Process Technol 124:165–173
Chen C, Yu J, Yoza BA et al (2015) Role and potential of spent fluid catalytic cracking catalyst assisted ozonation of petrochemical wastewater. J Environ Manag 152:58–65
Deng F, Qiu S, Chen C et al (2015) Heterogeneous Catalytic Ozonation of Refinery Wastewater over Alumina-Supported Mn and Cu Oxides Catalyst. Ozone-Sci Eng 37:546–555
Dong HH, Jiang X, Sun S et al (2019) A cascade of a denitrification bioreactor and an aerobic biofilm reactor for heavy oil refinery wastewater treatment. RSC Adv 9:7495–7504
El-Naas MH, Al-Zuhair S, Al-Lobaney A et al (2009) Assessment of electrocoagulation for the treatment of petroleum refinery wastewater. J Environ Manag 91:180–185
El-Naas MH, Alhaija MA, Al-Zuhair S (2014) Evaluation of a three-step process for the treatment of petroleum refinery wastewater. J Environ Chem Eng 2:56–62
Farré M, Ferrer I, Ginebreda A et al (2001) Determination of drugs in surface water and wastewater samples by liquid chromatography–mass spectrometry: methods and preliminary results including toxicity studies with Vibrio fischeri. J Chromatogr A 938:187–197
Feng M, Yan L, Zhang X et al (2016) Fast removal of the antibiotic flumequine from aqueous solution by ozonation: influence of factors, reaction pathways, and toxicity evaluation. Sci Total Environ 541:167–175
Hassani El K, Kalnina D, Maris T et al (2019) Enhanced degradation of an azo dye by catalytic ozonation over Ni-containing layered double hydroxide nanocatalysts. Sep Purif Technol 210:764–774
Hossain MZ, Wu W, Xu WZ et al (2018) High-surface-area mesoporous activated carbon from hemp bast fiber using hydrothermal processing. C 4(3):38–43
Islam MS, Ang BC, Gharehkani S et al (2016) Adsorption capacity of activated carbon synthesized from coconut shell. Carbon Lett 20:1–9
Ikhlaq A, Munir HMS, Khan A et al (2019) Comparative study of catalytic ozonation and Fenton-like processes using iron-loaded rice husk ash as catalyst for the removal of methylene blue in wastewater. Ozone-Sci Eng 41(3):250–260
Jiang SP (2006) A review of wet impregnation—an alternative method for the fabrication of high performance and nano-structured electrodes of solid oxide fuel cells. Mater Sci Eng A 418:199–210
Kermani M, Kakavandi B, Farzadkia M et al (2018) Catalytic ozonation of high concentrations of catechol over TiO2@Fe3O4 magnetic core-shell nanocatalyst: optimization, toxicity and degradation pathway studies. J Clean Prod 192:597–607
Luo Z (2011) The research of determination of sulfides by iodometric method. Guangzhou Chem Ind 12:113–115 (in Chinese)
Liu H, Chen L, Ji L (2019a) Ozonation of ammonia at low temperature in the absence and presence of MgO. J Hazard Mater 376:125–132
Lv A, Hu C, Nie Y et al (2010) Catalytic ozonation of toxic pollutants over magnetic cobalt and manganese co-doped γ-Fe2O3. Appl Catal B-Environ 100:62–67
Liu D, Wang C, Song Y, Wei Y, He L, Lan B, He X, Wang J (2019b) Effective mineralization of quinoline and bio-treated coking wastewater by catalytic ozonation using CuFe2O4/Sepiolite catalyst: efficiency and mechanism. Chemosphere 227:647–656
Munir HMS, Ferozea N, Ikhlaq A et al (2019) Removal of colour and COD from paper and pulp industry wastewater by ozone and combined ozone/UV process. Desalin Water Treat 137:154–161
Manivel A, Lee GJ, Chen CY et al (2015) Synthesis of MoO3 nanoparticles for azo dye degradation by catalytic ozonation. Mater Res Bull 62:184–191
Nawaz F, Cao H, Xie Y, Xiao J, Chen Y, Ghazi ZA (2017) Selection of active phase of MnO2 for catalytic ozonation of 4-nitrophenol. Chemosphere 168:1457–1466
Polat D, Balcı İ, Özbelge TA (2015) Catalytic ozonation of an industrial textile wastewater in a heterogeneous continuous reactor. J Environ Chem Eng 3:1860–1871
Rodriguez-Ruiz A, Etxebarria J, Boatti L, Marigomez I (2015) Scenariotargeted toxicity assessment through multiple endpoint bioassays in a soil posing unacceptable environmental risk according to regulatory screening values. Environ Sci Pollut Res 22:13344–13361
Raj KG, Joy PA (2015) Coconut shell based activated carbon-iron oxide magnetic nanocomposite for fast and efficient removal of oil gas. J Environ Chem Eng 3:2068–2075
Rodríguez-Reinoso F, Molina-Sabio M, Gonzalez MT (1995) The use of steam and CO as activating agents in the preparation of activated carbons. Carbon 33:15–23
Sania ON, Navaei fezabadya AA, Yazdanib M et al (2019) Catalytic ozonation of ciprofloxacin using γ-Al2O3 nanoparticles in synthetic and real wastewaters. J Water Process Eng 32:100894
Tian G, Wu Q, Li A, Wang W et al (2016) Promoted ozonation for the decomposition of 1, 4-dioxane by activated carbon. Water Sci Tech-Water Sup 17:613–620
Vittenet J, Aboussaoud W, Mendret J et al (2015) Catalytic ozonation with γ-Al2O3 to enhance the degradation of refractory organics in water. Appl Catal A-Gen 504:519–532
Wang Y, Cao H, Chen C et al (2019) Metal-free catalytic ozonation on surface-engineered graphene: microwave reduction and heteroatom doping. Chem Eng J 355:118–129
Xiong Y, He C, Karlsson HT, Zhu X (2003) Performance of three-phase three-dimensional electrode reactor for the reduction of COD in simulated wastewater-containing phenol. Chemosphere 50:131–136
Yang Y, Cao H, Peng P, Bo H (2014) Degradation and transformation of atrazine under catalyzed ozonation process with TiO2 as catalyst. J Hazard Mater 279:444–451
Zeng YF, Liu ZL, Qin ZZ (2009) Decolorization of molasses fermentation wastewater by SnO2-catalyzed ozonation. J Hazard Mater 162:682–687
Zhang Y, Cui F, Mu Y et al (2007) An improvement in quantitative determination of SO42− in oilfield produced water by EDTA complexometry. Oilfield Chem 4:369–371 (in Chinese)
Zhang T, Li CJ, Ma J et al (2008) Surface hydroxyl groups of synthetic a-FeOOH in promoting •OH generation from aqueous ozone: property and activity relationship. Appl Catal B-Environ 82:131–137
Zhang X, Li X, Qin W (2009) Investigation of the catalytic activity for ozonation on the surface of NiO nanoparticles. Chem Phys Lett 479:310–315
Funding
This work was supported by the open found of State Key Laboratory of Oil and Gas Reservoir Geology and Exploitation (grant no. PL1128) and the fund of the Back-bone of Young and Middle-age Personal Training Plan of Southwest Petroleum University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Vítor Pais Vilar
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 7018 kb)
Rights and permissions
About this article
Cite this article
Wang, W., Yao, H. & Yue, L. Supported-catalyst CuO/AC with reduced cost and enhanced activity for the degradation of heavy oil refinery wastewater by catalytic ozonation process. Environ Sci Pollut Res 27, 7199–7210 (2020). https://doi.org/10.1007/s11356-019-07410-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-07410-1