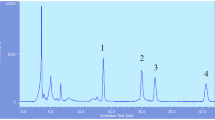
Abstract
Oily organic waste is a promising feedstock for anaerobic co-digestion. Free long-chain fatty acids (LCFAs) produced from lipids can inhibit methanogenic consortia, so optimal control of LCFA concentration is the key to successful operation of co-digestion. Most LCFAs are present in the solid phase, making them difficult to be detected and monitored. This study proposes a simple and easy method for detecting LCFAs in both the liquid and solid phases of anaerobic digestate by combining liquid–liquid extraction followed by solid-phase extraction (SPE) and spectrophotometric analysis. The extraction procedure successfully removed impurities that interfere with the absorbance spectrum and ensured high recovery rates of LCFAs. The utility of the pretreatment used for the extraction was discussed using thermodynamic analysis and calculations of phase equilibrium for the solvent extraction system. The absorbance spectrum shift of pyridinium N-phenolate betaine (PNPB) dye-stained solution showed a good correlation with LCFA concentration and enabled highly sensitive measurements. Good quantification was demonstrated in experiments using various digestate samples obtained from the laboratory, pilot, and full-scale reactors.





Similar content being viewed by others
References
Affes M, Aloui F, Hadrich F, Loukil S, Sayadi S (2017) Effect of bacterial lipase on anaerobic co-digestion of slaughterhouse wastewater and grease in batch condition and continuous fixed-bed reactor. Lipids Health Dis 16(1):195
Cavaleiro AJ, Salvador AF, Alves JI, Alves M (2009) Continuous high rate anaerobic treatment of oleic acid based wastewater is possible after a step feeding start-up. Environ Sci Technol 43(8):2931–2936
Dong T, Knoshaug EP, Pienkos PT, Laurens LML (2016) Lipid recovery from wet oleaginous microbial biomass for biofuel production: a critical review. Appl Energy 177:879–895
Douhal A (1994) Photophysics of Nile Blue A in proton-accepting and electron-donating solvents. Phys Chem 98(50):13131–13137
Flurkey WH (2005) Use of solid phase extraction in the biochemistry laboratory to separate different lipids. Biochem Mol Biol Educ 33(5):357–360
Fredenslund A, Jones RL, Prausnitz JM (1975) Group-contribution estimation of activity coefficients in non-ideal liquid mixtures. AICHE J 21:1086–1099
Jiney J, Kevin B (2006) Benzophenoxazine-based fluorescent dyes for labeling biomolecules. Tetrahedron. 62(48):11021
J. Hunter Long, Tarek N. Aziz, Francis L. de los Reyes, Joel J. Ducoste, (2012) Anaerobic co-digestion of fat, oil, and grease (FOG): A review of gas production and process limitations. Process Safety and Environmental Protection 90 (3):231-245
Kanicky JR, Shah DO (2002) Effect of degree, type, and position of unsaturation on the pKa of long-chain fatty acids. J Colloid Interface Sci 256(1):201–207
Kanicky JR, Poniatowski AF, Mehta NR, Shah DO (2000) Cooperativity among molecules at interfaces in relation to various technological processes: effect of chain length on the pKa of fatty acid salt solutions. Langmuir 16(1):172–177
Kirschman HD, Pomeroy R (1949) Determination of oil in oil-field waste waters. Anal Chem 21(7):793–797
Kleyböcker A, Liebrich M, Verstraete W, Kraume M, Würdemann H (2012) Early warning indicators for process failure due to organic overloading by rapeseed oil in one-stage continuously stirred tank reactor, sewage sludge and waste digesters. Bioresour Technol 123:534–541
Kobayashi T, Kuramochi H, Maeda K, Tsuji T, Xu KQ (2014) Dual-fuel production from restaurant grease trap waste: bio-fuel oil extraction and anaerobic methane production from the post-extracted residue. Bioresour Technol 169:134–142
Kobayashi T, Kuramochi H, Xu KQ (2017a) Variable oil properties and biome thane production of grease trap waste derived from different resources. Int Biodeterior Biodegrad 119:273–281
Kobayashi T, Kuramochi H, Maeda K, Xu KQ (2017b) A simple method for the detection of long-chain fatty acids in an anaerobic digestate using a quartz crystal sensor. Energies 10:19
Kubinyi M, Brátán J, Grofcsik A, Biczók L, Poór B, Bitter I, Grün A, Bogáti B, Tóth K (2002) Proton transfer and supramolecular complex formation between Nile blue and tetraundecylcalix[4]resorcinarene—a fluorescence spectroscopic study. J Chem Soc Perkin Trans 2:1784–1789
Kuramochi H, Maeda K, Kato S, Osako M, Nakamura K, Sakai S (2009) Application of UNIFAC models for prediction of vapor-liquid and liquid-liquid equilibria relevant to separation and purification processes of crude BDF. Fuel 88:1472–1477
Lalman JA, Bagley DM (2001) Anaerobic degradation and inhibitory effects of linoleic acid. Water Res 34(17):4220–4228
Li Y, Naghdi FG, Garg S, Adarme-Vega TC, Thurecht KJ, Ghafor WA, Tannock S, Schenk PM (2014) A comparative study: the impact of different lipid extraction methods on current microalgal lipid research. Microb Cell Factories 13:14
Linares P, Luque de Castro MD, Valcárcel M (1989) Direct automatic determination of free acidity in oils by flow-injection analysis. Anal Chim Acta 225:431–436
Luostarinen S, Luste S, Sillanpää M (2009) Increased biogas production at wastewater treatment plants through co-digestion of sewage sludge with grease trap sludge from a meat processing plant. Bioresour Technol 100:79–85
Magnussen T, Rasmussen P, Fredenslund A (1981) UNIFAC parameter table for prediction of liquid-liquid equilibria. Ind Eng Chem Process Des Dev 20:331–339
Mariotti E, Mascini M (2001) Determination of extra virgin olive oil acidity by FIA-titration. Food Chem 73:235–238
M.A. Pereira, A.J. Cavaleiro, M. Mota, M.M. Alves, (2003) Accumulation of long chain fatty acids onto anaerobic sludge under steady state and shock loading conditions: effect on acetogenic and methanogenic activity. Water Science and Technology 48 (6):33-40
Neves L, Pereira MA, Mota M, Alves MM (2009) Detection and quantification of long chain fatty acids in liquid and solid samples and its relevance to understand anaerobic digestion of lipids. Bioresour Technol 100(1):91–96
Oren JJ, MacKay GDM (1977) Electrolyte and pH effect on emulsion stability of water-in-petroleum oils. Fuel 56(4):382–384
Paul GW, Marc de Chazal LE (1967) Interfacial tensions in ternary liquid-liquid systems. J Chem Eng Data 12(1):105–107
Pereira MA, Pires OC, Mota M, Alves MM 2005(2005) Anaerobic biodegradation of oleic and palmitic acids: evidence of mass transfer limitations caused by long chain fatty acid accumulation onto the anaerobic sludge. Biotechnol Bioeng 92(1):15–23
Reichardt C (1994) Solvatochromic dyes as solvent polarity indicators. Chem Rev 94(8):2319–2358
Salama ES, Saha S, Kurade MB, Dev S, Chang SW, Jeon BH (2019) Recent trends in anaerobic co-digestion: fat, oil, and grease (FOG) for enhanced biomethanation. Prog Energy Combust Sci 70:22–42
Schwarzenbach RP, Gschwend PM, Imboden DM (2003) Environmental organic chemistry, 2nd edn. John Wiley, Hoboken
Sousa DZ, Salvador AF, Ramos J, Guedes AP, Barbosa S, Stams AJ, Alves MM, Pereira MA (2013) Activity and viability of methanogens in anaerobic digestion of unsaturated and saturated long-chain fatty acids. Appl Environ Microbiol 79(14):4239–4245
Sun SY, Wang D, Yan J, Qiao W, Wang W, Zhu T (2014) Effects of lipid concentration on anaerobic co-digestion of municipal biomass wastes. Waste Manag 34:1025–1034
Wu LJ, Kobayashi T, Kuramochi H, Li YY, Xu KQ, Lv Y (2018) High loading anaerobic co-digestion of food waste and grease trap waste: determination of the limit and lipid/long chain fatty acid conversion. Chem Eng J 338:422–431
Yau YH, Rudolph V, Lo CC, Wu KC (2018) Restaurant oil and grease management in Hong Kong. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-018-2474-4
Acknowledgments
We would like to thank Mr. Yuji Yamazaki, Tomoyuki Nara, and Toshitaka Kato of Takenaka Corporation, Japan, for their valuable help in the collection of digestate samples.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Ta Yeong Wu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 137 kb)
Rights and permissions
About this article
Cite this article
Kobayashi, T., Kuramochi, H., Xu, KQ. et al. Simple solvatochromic spectroscopic quantification of long-chain fatty acids for biological toxicity assay in biogas plants. Environ Sci Pollut Res 27, 17596–17606 (2020). https://doi.org/10.1007/s11356-019-06532-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06532-w