Abstract
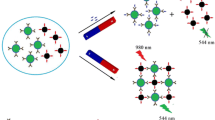
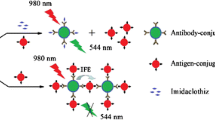
A rapid and sensitive immunoassay for the simultaneous detection of imidacloprid and thiacloprid was developed by using magnetic nanoparticles (MNPs) and upconversion nanoparticles (UCNPs). The UCNPs of NaYF4:Yb, Er and NaYF4:Yb, Tm were synthesized and conjugated with anti-imidacloprid monoclonal antibody (mAb) and anti-thiacloprid mAb as signal labels, while the MNPs were conjugated with antigens of thiacloprid and imidacloprid as separation elements. The fluorescence intensities of Yb/Er- and Yb/Tm-doped UCNPs were detected simultaneously in 544 nm and 477 nm under the excitation of NIR light (980 nm). The amounts of mAb-conjugated UCNPs that were separated by antigen-conjugated MNPs were determined based on competitive immunoassays. Under the optimal conditions, the 50% inhibiting concentration (IC50) and limit of detection (LOD, IC10) were 5.80 and 0.32 ng/mL for imidacloprid and 6.45 and 0.61 ng/mL for thiacloprid, respectively. The immunoassay exhibited negligible cross-reactivity with analogs of imidacloprid and thiacloprid except imidaclothiz (86.2%). The average recoveries of imidacloprid and thiacloprid in environmental and agricultural samples, including paddy water, soil, pears, oranges, cucumbers, and wheat, ranged from 78.4 to 105.9% with relative standard deviations (RSDs) of 2.1–11.9% for imidacloprid and ranged from 82.5 to 102.3% with RSDs of 1.0–16.5% for thiacloprid. In addition, the results of the immunoassay correlated well with high-performance liquid chromatography for the detection of the authentic samples.




Similar content being viewed by others
References
Baskaran S, Kookana RS, Naidu R (1997) Determination of the insecticide imidacloprid in water and soil using high-performance liquid chromatography. J Chromatogr A 787:271–275
Chen H, Yang Q, Ding Y, Vasylieva N, Bever CS, Hua XD, Wang MH, Hammock BD (2019) Competitive and noncompetitive immunoassays for the detection of benzothiostrobin using magnetic nanoparticles and fluorescein isothiocyanate-labeled peptides. Anal Bioanal Chem 411:527–535
Eisenback BM, Mullins DE, Salom SM, Kok LT (2009) Evaluation of ELISA for imidacloprid detection in eastern hemlock (Tsuga canadensis) wood and needle tissues. Pest Manag Sci 65:122–128
Elbert A, Haas M, Springer B, Thielert W, Nauen R (2008) Applied aspects of neonicotinoid uses in crop protection. Pest Manag Sci 64:1099–1105
Girotti S, Maiolini E, Ghini S, Eremin S, Mañes J (2010) Quantification of imidacloprid in honeybees: development of a chemiluminescent ELISA. Anal Lett 43:466–475
Hua XD, You HJ, Luo PW, Tao ZX, Chen H, Wang MH (2017) Upconversion fluorescence immunoassay for imidaclothiz by magnetic nanoparticle separation. Anal Bioanal Chem 409:1–8
Henry M, Beguin M, Requier F, Rollin O, Odoux J, Aupinel P, Aptel J, Tchamitchian S, Decourtye A (2012) A common pesticide decreases foraging success and survival in honey bees. Science 336:348–350
Ishii Y, Kobori I, Araki Y, Kurogochi S, Iwaya JK, Kagabug S (1994) HPLC determination of the new insecticide imidacloprid and its behavior in rice and cucumber. J Agr Food Chem 42:2917–2921
Kaur N, Sohal BS, Singh K (2011) Biochemical and physiological changes on bacillus thuringiensis cotton after imidacloprid foliar spray. Pestic Biochem Phys 99:280–284
Kim HJ, Shelver WL, Li QX (2004) Monoclonal antibody-based enzyme-linked immunosorbent assay for the insecticide imidacloprid. Anal Chim Acta 509:111–118
Liu ZJ, Wei X, Xu H, Li M, Zhu GB, Xue YL, Zhang Z, Zhao GD, Du DL (2016) Sensitive detection of thiacloprid in environmental and food samples by enhanced chemiluminescent enzyme immunoassay. RSC Adv 6:29460–29465
Liu ZJ, Yan X, Hua XD, Wang MH (2013) Time-resolved fluoroimmunoassay for quantitative determination of thiacloprid in agricultural samples. Anal Methods 5:3572–3576
Lu HC, Yi GS, Zhao SY, Chen DP, Guo LH, Cheng J (2004) Synthesis and characterization of multi-functional nanoparticles possessing magnetic, up-conversion fluorescence and bio-affinity properties. J Mater Chem 14:1336–1341
Luo C, Jones CM, Devine G, Zhang F, Denholm I, Gorman K (2010) Insecticide resistance in Bemisia tabaci biotype Q (Hemiptera Aleyrodide) from China. Crop Prot 29:429–434
MacDonald LM, Meyer TR (1998) Determination of imidacloprid and triadimefon in white pine by gas chromatography/mass spectrometry. J Agr Food Chem 46:3133–3138
Nakanishi K, Yokomizo H, Hayashi TI (2018) Were the sharp declines of dragonfly populations in the 1990s in Japan caused by fipronil and imidacloprid? An analysis of Hill’s causality for the case of Sympetrum frequens. Environ Sci Pollut Res 25:35352–35364
Navalon A, Gonzalez-Casado A, ElKhattabi R, Vilchez JL, Fernandezalba AR (1997) Determination of imidacloprid in vegetable samples by gas chromatography–mass spectrometry. Analyst 122:579–581
Qiu PY, Zhou N, Chen HY, Zhang CL, Gao G, Cui DX (2013) Recent advances of lanthanide-doped upconversion nanomaterials: synthesis, nanostructures and surface modification. Nanoscale 5:11512–11525
Shi HY, Sheng EZ, Feng L, Zhou LL, Hua XD, Wang MH (2015) Simultaneous detection of imidacloprid and parathion by the dual-labeled time-resolved fluoroimmunoassay. Environ Sci Pollut Res 22:14882–14890
Si FF, Zou RB, Jiao SS, Qiao XS, Guo YR, Zhu GN (2018) Inner filter effect-based homogeneous immunoassay for rapid detection of imidacloprid residue in environmental and food samples. Ecotox Environ Safe 148:862–868
Stalder K, Stöber W (1965) Haemolytic activity of suspensions of different silica modifications and inert dusts. Nature 999:874–875
Sun NN, Ding Y, Tao ZX, You HJ, Hua XD, Wang MH (2018) Development of an upconversion fluorescence DNA probe for the detection of acetamiprid by magnetic nanoparticles separation. Food Chem 257:289–294
Tomizawa M, Casida JE (2005) Neonicotinoid insecticide toxicology: mechanisms of selective action. Annu Rev Pharmacol 45:247–268
Wang F, Tan W, Zhang Y, Fan X, Wang M (2005) TOPICAL REVIEW: Luminescent nanomaterials for biological labeling. Nanotechnology 17:R1–R13
Wang M, Abbineni G, Clevenger A, Mao CB, Xu SK (2011) Upconversion nanoparticles: synthesis, surface modification and biological applications. Nanomed Nanotechnol 7:710–729
Watanabe E, Seike N, Motoki Y, Inao K, Otani T (2016) Potential application of immunoassays for simple, rapid and quantitative detections of phytoavailable neonicotinoid insecticides in cropland soils. Ecotox Environ Safe 132:288–294
Whitehorn PR, O’Connor S, Wackers FL, Goulson D (2012) Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 336:351–352
Wu SJ, Duan N, Zhu CQ, Ma XY, Wang M, Wang ZP (2011) Magnetic nanobead-based immunoassay for the simultaneous detection of aflatoxin B1 and ochratoxin a using upconversion nanoparticles as multicolor labels. Biosens Bioelectron 30:35–42
Xu H, Zhang ZJ (2007) Functionalized fluorescent core-shell nanoparticles used as a fluorescent labels in fluoroimmunoassay for IL-6. Biosens Bioelectron 22:2743–2748
Xu T, Xu Q, Li H, Wang J, Li QX, Shelver WL, Li J (2012) Strip-based immunoassay for the simultaneous detection of the neonicotinoid insecticides imidacloprid and thiamethoxam in agricultural products. Talanta 101:85–90
Yang JC, Yang Q, Deng JQ, Tao ZX, Hua XD, Wang MH (2018) Development of immunochromatographic assays for the detection of imidacloprid in soil chemical barrier. Environ Sci Pollut Res 25:26617–26624
Yang Y (2014) Upconversion nanophosphors for use in bioimaging, therapy, drug delivery and bioassays. Microchim Acta 181:263–294
Yi GS, Chow GM (2006) Synthesis of hexagonal-phase NaYF4:Yb,Er and NaYF4:Yb,tm nanocrystals with efficient up-conversion fluorescence. Adv Funct Mater 16:2324–2329
Yin W, Hua XD, Liu XF, Shi HY, Shirley JG, Wang MH, Bruce DH (2015) Development of an enzyme-linked immunosorbent assay for thiacloprid in soil and agro-products with phage-displayed peptide. Anal Biochem 481:27–32
You HJ, Hua XD, Feng L, Sun NN, Rui Q, Wang LM, Wang MH (2017) Competitive immunoassay for imidaclothiz using upconversion nanoparticles and gold nanoparticles as labels. Microchim Acta 184:1085–1092
Zhao JW, Sun YJ, Kong XG, Tian LJ, Wang Y, Tu LP, Zhao JL, Zhang H (2008) Controlled synthesis, formation mechanism, and great enhancement of red upconversion luminescence of NaYF4: Yb3+, Er3+ nanocrystals/submicroplates at low doping level. J Phys Chem B 112:15666–15672
Funding
This work was supported by the National Key Research and Development Program of China (2017YFF0210200), the Fundamental Research Funds for the Central Universities (KYZ201618), and the National Natural Science Foundation of China (31772194).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 2025 kb)
Rights and permissions
About this article
Cite this article
Tao, Z., Deng, J., Wang, Y. et al. Competitive immunoassay for simultaneous detection of imidacloprid and thiacloprid by upconversion nanoparticles and magnetic nanoparticles. Environ Sci Pollut Res 26, 23471–23479 (2019). https://doi.org/10.1007/s11356-019-05635-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05635-8