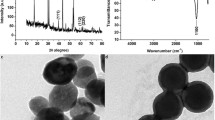
Abstract
Two high-sensitivity competitive immune-nanoplatforms based on the inner filter effect (IFE-IN) and magnetic separation (MS-IN) with a positive readout were developed to rapidly detect imidacloprid (IMI) using gold nanoparticles (AuNPs). For IFE-IN, IMI competes with AuNPs-labeled IMI antigens (IMI-BSA-AuNPs) to bind with anti-IMI monoclonal antibody (mAb)-conjugated NaYF4:Yb,Er upconversion nanoparticles, which changes the fluorescence signal at excitation/emission wavelength of 980/544 nm. For MS-IN, the immunocomplex of IMI-BSA-AuNPs and magnetic-nanoparticles-labeled mAb (mAb-MNPs) dissociates in the presence of IMI, and the optical density of IMI-BSA-AuNPs at 525 nm increases with the IMI concentration after magnetic separation. Under the optimal conditions, the IMI concentration producing a 50% saturation of the signal (SC50) and linear range (SC10− SC90) were found to be 4.30 ng mL−1 and 0.47 − 21.37 ng mL−1 for IFE-IN, while 1.21 ng mL−1 and 0.07 − 10.21 ng mL−1 for MS-IN, respectively. Both IFE-IN and MS-IN achieved excellent accuracy for the detection of IMI in different matrices. The quantities of IMI in apple samples detected by IFE-IN and MS-IN were consistent with the high-performance liquid chromatography results.
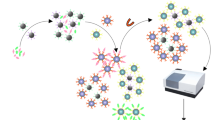
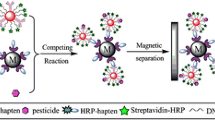
Graphical abstract
For IFE-IN, analyte competes with AuNPs-labeled-antigen to bind with the mAb-conjugated-UCNPs, which changes the fluorescence signal at 544 nm. For MS-IN, the immunocomplex of AuNPs-labeled-antigen and mAb-conjugated-MNPs dissociates in the presence of analyte, and the optical density of AuNPs-labeled-antigen at 525 nm increases with increasing analyte concentration after separation.






Similar content being viewed by others
References
Fang QK, Wang LM, Cheng Q, Cai J, Wang YL, Yang MM, Hua XD, Liu FQ (2015) A bare-eye based one-step signal amplified semiquantitative immunochromatographic assay for the detection of imidacloprid in Chinese cabbage samples. Anal Chim Acta 881:82–89. https://doi.org/10.1016/j.aca.2015.04.047
Xu T, Xu QG, Li H, Wang J, Li QX, Shelver WL, Li J (2012) Strip-based immunoassay for the simultaneous detection of the neonicotinoid insecticides imidacloprid and thiamethoxam in agricultural products. Talanta 101:85–90. https://doi.org/10.1016/j.talanta.2012.08.047
Decourtye A, Devillers J (2010) Ecotoxicity of neonicotinoid insecticides to bees. Adv Exp Med Biol 683:85–95. https://doi.org/10.1007/978-1-4419-6445-8_8
Bandeira FO, Alves PRL, Hennig TB, Schiehl A, Cardoso EJBN, Baretta D (2020) Toxicity of imidacloprid to the earthworm Eisenia andrei and collembolan Folsomia candida in three contrasting tropical soils. J Soil Sediment 20:1997–2007. https://doi.org/10.1007/s11368-019-02538-6
Kim S, Lee HS, Park Y (2017) Perinatal exposure to low-dose imidacloprid causes ADHD-like symptoms: evidences from an invertebrate model study. Food Chem Toxicol 110:402–407. https://doi.org/10.1016/j.fct.2017.10.007
Tao ZX, Deng JQ, Wang Y, Chen H, Ding Y, Hua XD, Wang MH (2019) Competitive immunoassay for simultaneous detection of imidacloprid and thiacloprid by upconversion nanoparticles and magnetic nanoparticles. Environ Sci Pollut R 26:23471–23479. https://doi.org/10.1007/s11356-019-05635-8
Lee KL, You ML, Tsai CH, Lin EH, Hsieh SY, Ho MH, Hsu JC, Wei PK (2016) Nanoplasmonic biochips for rapid label-free detection of imidacloprid pesticides with a smartphone. Biosens Bioelectron 75:88–95. https://doi.org/10.1016/j.bios.2015.08.010
Askaran S, Kookana RS, Naidu R (1997) Determination of the insecticide imidacloprid in water and soil using high-performance liquid chromatography. J Chromatogr A 787:271–275. https://doi.org/10.1016/S0021-9673(97)00652-3
Blasco C, Fernadez M, Pico Y, Font G, Manes J (2002) Simultaneous determination of imidacloprid carbevdazim, methiocarb and hexythiazox in peaches and nectarines by liquid chromatography-mass spectrometry. Anal Chim Acta 461:109–116. https://doi.org/10.1016/S0003-2670(02)00255-6
Cavalera S, Colitti B, Rosati S, Ferrara G, Bertolotti L, Nogarol C, Guiotto C, Cagnazzo C, Denina M, Fagioli F, Di Nardo F, Chiarello M, Baggiani C, Anfossi L (2021) A multi-target lateral flow immunoassay enabling the specific and sensitive detection of total antibodies to SARS COV-2. Talanta 223:121737. https://doi.org/10.1016/j.talanta.2020.121737
Wang ZH, Yao XL, Zhang YZ, Wang R, Ji YW, Sun J, Zhang DH, Wang JL (2020) Functional nanozyme mediated multi-readout and label-free lateral flow immunoassay for rapid detection of Escherichia coli O157:H7. Food Chem 329:127224. https://doi.org/10.1016/j.foodchem.2020.127224
Wang YN, Zhang K, Huang XD, Qiao L, Liu BH (2021) Mass spectrometry imaging of mass tag immunoassay enables the quantitative profiling of biomarkers from dozens of exosomes. Anal Chem 93:709–714. https://doi.org/10.1021/acs.analchem.0c03904
Hao LW, Chen J, Chen XR, Ma TT, Cai XX, Duan H, Leng YK, Huang XL, Xiong YH (2021) A novel magneto-gold nanohybrid-enhanced lateral flow immunoassay for ultrasensitive and rapid detection of ochratoxin A in grape juice. Food Chem 33:127710. https://doi.org/10.1016/j.foodchem.2020.127710
Zhang XY, Zhang XJ, Song LJ, Huang XQ, Li Y, Qiao MW, Liu WJ, Zhang TT, Qi YC, Wang WZ, Yu XZ, Dou LN, Yang HJ, Wang LY, Mao YX, Wang ZH (2021) An ultrasensitive, homogeneous fluorescence quenching immunoassay integrating separation and detection of aflatoxin M-1 based on magnetic graphene composites. Microchim Acta 188:59. https://doi.org/10.1007/s00604-021-04715-2
Zhang GG, Lai XC, Ding NS, Xiong QR, Hou S, Duan HW, Lai WH (2021) Chrysanthemum-like Au@Polydopamine synthesized using one-pot method and its advantage in immunochromatographic assay. Sensor Actuat B-Chem 343:130097. https://doi.org/10.1016/j.snb.2021.130097
Gonzalez-Techera A, Kim HJ, Gee SJ, Last JA, Hammock BD, Gonzalez-Sapienza G (2007) Polyclonal antibody-based noncompetitive immunoassay for small analytes developed with short peptide loops isolated from phage libraries. Anal Chem 79:9191–9196. https://doi.org/10.1021/ac7016713
Lim SL, Ichinose H, Shinoda T, Ueda H (2007) Noncompetitive detection of low molecular weight peptides by open sandwich immunoassay. Anal Chem 79:6193–6200. https://doi.org/10.1021/ac070653z
Hua XD, Zhou LL, Feng L, Ding Y, Shi HY, Wang LM, Gee SJ, Hammock BD, Wang MH (2015) Competitive and noncompetitive phage immunoassays for the determination of benzothiostrobin. Anal Chim Acta 890:150–156. https://doi.org/10.1016/j.aca.2015.07.056
Cardozo S, Gonzalez-Techera A, Last JA, Hammock BD, Kramer K, Gonzalez-Sapienza G (2005) Analyte peptidomimetics selected from phage display peptide libraries: a systematic strategy for the development of environmental immunoassays. Environ Sci Technol 39:4234–4241. https://doi.org/10.1021/es0479311
Kim HJ, Gonzalez-Techera A, Gonzalez-Sapienza G, Ahn KC, Gee SJ, Hammock BD (2008) Phage-borne peptidomimetics accelerate the development of polyclonal antibody-based heterologous immunoassays for the detection of pesticide metabolites. Environ Sci Technol 42:2047–2053. https://doi.org/10.1021/es702219a
Ding Y, Hua XD, Chen H, Liu FQ, Gonzalez-Sapienza G, Wang MH (2018) Recombinant peptidomimetic-nano luciferase tracers for sensitive single-step immunodetection of small molecules. Anal Chem 90:2230–2237. https://doi.org/10.1021/acs.analchem.7b04601
Misawa K, Yamamoto T, Hiruta Y, Yamazaki H, Citterio D (2020) Text-displaying semiquantitative competitive lateral flow immunoassay relying on inkjet-printed patterns. ACS Sensors 5:2076–2085. https://doi.org/10.1021/acssensors.0c00637
Lassabe G, Rossotti M, Gonzalez-Techera A, Gonzalez-Sapienza G (2014) Shiga-like toxin B subunit of Escherichia coli as scaffold for high-avidity display of anti-immunocomplex peptides. Anal Chem 86:5541–5546. https://doi.org/10.1021/ac500926f
Vanrell L, Gonzalez-Techera A, Hammock BD, Gonzalez-Sapienza G (2013) Nanopeptamers for the development of small-analyte lateral flow tests with a positive readout. Anal Chem 85:1177–1182. https://doi.org/10.1021/ac3031114
Shi CY, Deng N, Liang JJ, Zhou KN, Fu QQ, Tang Y (2015) A fluorescent polymer dots positive readout fluorescent quenching lateral flow sensor for ractopamine rapid detection. Anal Chim Acta 854:202–208. https://doi.org/10.1016/j.aca.2014.11.005
Chen H, Ding Y, Yang Q, Barnych B, Gonzalez-Sapienza G, Hammock BD, Wang MH, Hua XD (2019) Fluorescent “turn off-on” small-molecule-monitoring nanoplatform based on dendrimer-like peptides as competitors. ACS Appl Mater Inter 11:33380–33389. https://doi.org/10.1021/acsami.9b13111
Porizka P, Vytiskova K, Oborilova R, Pastucha M, Gabris I, Brandmeier JC, Modlitbova P, Gorris HH, Novotny K, Skladal P, Kaiser J, Farka Z (2021) Laser-induced breakdown spectroscopy as a readout method for immunocytochemistry with upconversion nanoparticles. Microchim Acta 188:147. https://doi.org/10.1007/s00604-021-04816-y
Si FF, Zou RB, Jiao SS, Qiao XS, Guo YR, Zhu GN (2018) Inner filter effect-based homogeneous immunoassay for rapid detection of imidacloprid residue in environmental and food samples. Ecotox Environ Safe 148:862–868. https://doi.org/10.1016/j.ecoenv.2017.11.062
Sun Y, Li YS, Meng XM, Meng XY, Qin B, Si ZZ, Hu B, Lu SY, Ren HL, Liu ZS, Zhang Y, Meng L, Zhou Y (2017) Magnetic nanoparticles-phenanthrene conjugates (MNPs-Phe) probe based competitive chemiluminescence enzyme immunoassay (MNPs-icCLEIA) for phenanthrene and other homologous polycyclic aromatic hydrocarbons. Sensor Actuat B-Chem 252:633–640. https://doi.org/10.1016/j.snb.2017.06.056
Diaz-Marta AS, Yanez S, Tubio CR, Barrio VL, Pineiro Y, Pedrido R, Rivas J, Amorin M, Guitian F, Coelho A (2019) Multicatalysis combining 3D-printed devices and magnetic nanoparticles in one-pot reactions: steps forward in compartmentation and recyclability of catalysts. ACS Appl Mater Inter 11:25283–25294. https://doi.org/10.1021/acsami.9b08119
Xiong Y, Fu T, Zhang DX, Zhang SP, Xu HX (2021) Superradiative plasmonic nanoantenna biosensors enable sensitive immunoassay using the naked eye. Nanoscale 13:2429–2435. https://doi.org/10.1039/d0nr06148d
Yao S, Li J, Pang B, Wang XC, Shi YJ, Song XL, Xu K, Wang J, Zhao C (2020) Colorimetric immunoassay for rapid detection of Staphylococcus aureus based on etching-enhanced peroxidase-like catalytic activity of gold nanoparticles. Microchim Acta 187:9. https://doi.org/10.1007/s00604-020-04473-7
Yang JC, Yang Q, Deng JQ, Tao ZX, Hua XD, Wang MH (2018) Development of immunochromatographic assays for the detection of imidacloprid in soil chemical barrier. Environ Sci Pollut R 25:26617–26624. https://doi.org/10.1007/s11356-018-2707-6
Hua XD, You HJ, Luo PW, Tao ZX, Chen H, Liu FQ, Wang MH (2017) Upconversion fluorescence immunoassay for imidaclothiz by magnetic nanoparticle separation. Anal Bioanal Chem 409:6885–6892. https://doi.org/10.1007/s00216-017-0653-7
Mackenzie LE, Goode JA, Vakurov A, Nampi PP, Saha S, Jose G, Millner PA (2018) The theoretical molecular weight of NaYF4:RE upconversion nanoparticles. Sci Rep 8:1106. https://doi.org/10.1038/s41598-018-19415-w
Frens G (1973) Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat Phys Sci 241:20–22. https://doi.org/10.1038/physci241020a0
Samhadaneh DM, Chu SW, Maysinger D, Stochaj U (2020) How could gold nanourchins be applied in the clinic? Nanomedicine 15:829–832. https://doi.org/10.2217/nnm-2019-0438
Nieves RA, Ellis RP, Todd RJ, Johnson TJA, Grohmann K, Himmel ME (1991) Visualization of Trichoderma reesei cellobiohydrolase I and endoglucanase ion aspen cellulose by using monoclonal antibody-colloidal gold conjugates. Appl Environ Microbiol 11:3163–3170. https://doi.org/10.1002/yea.320070912
Zhang LN, Wang ZF, Wen YB, Shi JW, Wang JC (2015) Simultaneous detection of parathion and imidacloprid using broad-specificity polyclonal antibody in enzyme-linked immunosorbent assay. Anal Methods 7:205–210. https://doi.org/10.1039/c4ay01818d
Wang LM, Cai J, Wang YL, Fang QK, Wang SY, Cheng Q, Du D, Lin YH, Liu FQ (2014) A bare-eye-based lateral flow immunoassay based on the use of gold nanoparticles for simultaneous detection of three pesticides. Microchim Acta 181:1565–1572. https://doi.org/10.1007/s00604-014-1247-0
Funding
This work was funded by the National Key Research and Development Program of China (2017YFF0210200), the Fundamental Research Funds for the Central Universities (KYZ201618), and the National Natural Science Foundation of China (31772194).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, H., Sun, W., Zhang, Z. et al. Competitive immune-nanoplatforms with positive readout for the rapid detection of imidacloprid using gold nanoparticles. Microchim Acta 188, 356 (2021). https://doi.org/10.1007/s00604-021-05027-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-05027-1