Abstract
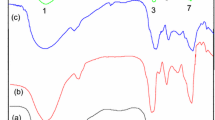
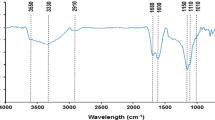
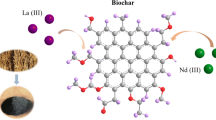
In order to remove aqueous radionuclides and find an appropriate method for the disposal of wild duckweed in eutrophic water body, alkali-treated duckweed biomass and duckweed-based hydrothermal biochar (hydrochar) and pyrolytic biochars of 300 and 600 °C were prepared. Their physicochemical properties were characterized carefully. The adsorption isothermal data fitted well with the Langmuir model and the maximum Langmuir adsorption capacities were 104.1, 96.3, 86.7, and 63.5 mg/g for hydrochar, modified biomass, and 300 and 600 °C biochars, respectively. The adsorption kinetic data fitted well with the pseudo-second-order kinetic equation. The sorption data of fixed-bed column also confirmed the high efficient removal of Th(IV) and fitted well with the Thomas model. The duckweed-based hydrothermal biochar is a low-cost adsorbent for Th(IV) removal, and it is also a resource utilization technology of the duckweed collected from eutrophic water body.





Similar content being viewed by others
References
Abd El-Latif MM, Elkady MF (2010) Equilibrium isotherms for harmful ions sorption using nano zirconium vanadate ion exchanger. Desalination 255:21–43
Abubakar Sadiq A, Umar I, Chidozie Timothy A, Nuraddeen Nasiru G, Ahmad Termizi R (2015) Health and ecological hazards due to natural radioactivity in soil from mining areas of Nasarawa state, Nigeria. Isot Environ Health Stud 51:448–468
Ahmad M, Rajapaksha AU, Lim JE, Zhang M, Bolan N, Mohan D, Vithanage M, Lee SS, Ok YS (2014) Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere 99:19–33
Anirudhan TS, Sreekumari SS, Jalajamony S (2013) An investigation into the adsorption of thorium(IV) from aqueous solutions by a carboxylate-functionalised graft copolymer derived from titanium dioxide-densified cellulose. J Environ Radioact 116:141–147
Atta AM, Akl ZF (2015) Removal of thorium from water using modified magnetite nanoparticles capped with rosin amidoxime. Mater Chem Phys 163:253–261
Chen C, Zhou W, Lin D (2015) Sorption characteristics of N-nitrosodimethylamine onto biochar from aqueous solution. Bioresour Technol 179:359–366
Chia CH, Gong B, Joseph SD, Marjo CE, Munroe P, Rich AM (2012) Imaging of mineral-enriched biochar by FTIR, Raman and SEM–EDX. Vib Spectrosc 62:248–257
Demirbas A (2004) Effects of temperature and particle size on bio-char yield from pyrolysis of agricultural residues. J Anal Appl Pyrolysis 72:243–248
Ding Z, Wan Y, Hu X, Wang S, Zimmerman AR, Gao B (2016) Sorption of lead and methylene blue onto hickory biochars from different pyrolysis temperatures: importance of physicochemical properties. J Ind Eng Chem 37:261–267
Ding ZH, Wu HL, Hu X (2017) Multiple characterization for mechanistic insights of Pb(II) sorption onto biochars derived from herbaceous plant, biosolid, and livestock waste. Bioresources 12:6763–6772
Ertaş M, Alma MH (2010) Pyrolysis of laurel (Laurus nobilis L.) extraction residues in a fixed-bed reactor: characterization of bio-oil and bio-char. J Anal Appl Pyrolysis 88:22–29
Garlapalli RK, Wirth B, Reza MT (2016) Pyrolysis of hydrochar from digestate: effect of hydrothermal carbonization and pyrolysis temperatures on pyrochar formation. Bioresour Technol 220:168–174
Guo X, Zhang S, Shan XQ (2008) Adsorption of metal ions on lignin. J Hazard Mater 151:134–142
Hernandez-Mena LE, Pecora AAB, Beraldo AL (2014) Slow pyrolysis of bamboo biomass: analysis of biochar properties. Chem Eng Trans 37:115–120
Hu X, Ding ZH, Zimmerman AR, Wang SS, Gao B (2015) Batch and column sorption of arsenic onto iron-impregnated biochar synthesized through hydrolysis. Water Res 68:206–216
Huang YJ, Chen CF, Huang YC, Yue QJ, Zhong CM, Tan CJ (2015) Natural radioactivity and radiological hazards assessment of bone-coal from a vanadium mine in Central China. Radiat Phys Chem 107:82–88
Humelnicu D, Drochioiu G, Popa K (2004) Bioaccumulation of thorium and uranyl ions on Saccharomyces cerevisiae. J Radioanal Nucl Chem 260:291–293
Inyang MI, Gao B, Yao Y, Xue YW, Zimmerman A, Mosa A, Pullammanappallil P, Ok YS, Cao XD (2016) A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Crit Rev Environ Sci Technol 46:406–433
Jiang ZH, Yang Z, So CL, Hse CY (2007) Rapid prediction of wood crystallinity in Pinus elliotii plantation wood by near-infrared spectroscopy. J Wood Sci 53:449–453
Jiménez-Cedillo MJ, Olguín MT, Fall C, Colin-Cruz A (2013) As(III) and as(V) sorption on iron-modified non-pyrolyzed and pyrolyzed biomass from Petroselinum crispum (parsley). J Environ Manag 117:242–252
Jindo K, Mizumoto H, Sawada Y, Sanchezmonedero MA, Sonoki T (2014) Physical and chemical characterization of biochars derived from different agricultural residues. Biogeosci Discuss 11:6613–6621
Kaygun AK, Akyil S (2007) Study of the behaviour of thorium adsorption on PAN/zeolite composite adsorbent. J Hazard Mater 147:357–362
Kazy SK, D'Souza SF, Sar P (2009) Uranium and thorium sequestration by a Pseudomonas sp.: mechanism and chemical characterization. J Hazard Mater 163:65–72
Keiluweit M, Nico PS, Johnson MG, Kleber M (2010) Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ Sci Technol 44:1247–1253
Kim KH, Kim JY, Cho TS, Choi JW (2012) Influence of pyrolysis temperature on physicochemical properties of biochar obtained from the fast pyrolysis of pitch pine (Pinus rigida). Bioresour Technol 118:158–162
Kosmulski M (2009) pH-dependent surface charging and points of zero charge. IV. Update and new approach. J Colloid Interface Sci 337:439–448
Kutahyali C, Eral M (2010) Sorption studies of uranium and thorium on activated carbon prepared from olive stones: kinetic and thermodynamic aspects. J Nucl Mater 396:251–256
Lee SJ, Jin HP, Ahn YT, Chung JW (2015) Comparison of heavy metal adsorption by Peat Moss and Peat Moss-derived biochar produced under different carbonization conditions. Water Air Soil Pollut 226(2):1–11
Mumme J, Eckervogt L, Pielert J, Diakité M, Rupp F, Kern J (2011) Hydrothermal carbonization of anaerobically digested maize silage. Bioresour Technol 102:9255–9260
Rana D, Matsuura T, Kassim MA, Ismail AF (2013) Radioactive decontamination of water by membrane processes — a review. Desalination 321:77–92
Robertson J (2004) Raman spectroscopy of amorphous, nanostructured, diamond-like carbon, and Nanodiamond. Philos Trans Math Phys Eng Sci 362:2477–2512
Ronsse F, Sv H, Dickinson D, Prins W (2013) Production and characterization of slow pyrolysis biochar: influence of feedstock type and pyrolysis conditions. Glob Change Biol Bioenergy 5:104–115
Salinas-Pedroza MG, Olguín MT (2004) Thorium removal from aqueous solutions of Mexican erionite and X zeolite. J Radioanal Nucl Chem 260:115–118
Shin HS, Kim JH (2016) Isotherm, kinetic and thermodynamic characteristics of adsorption of paclitaxel onto Diaion HP-20. Process Biochem 51:917–924
Smith MW, Dallmeyer I, Johnson TJ, Brauer CS, Mcewen JS, Espinal JF, Garcia-Perez M (2016) Structural analysis of char by Raman spectroscopy: improving band assignments through computational calculations from first principles. Carbon 100:678–692
Uchimiya M, Wartelle LH, Klasson KT, Fortier CA, Lima IM (2011) Influence of pyrolysis temperature on biochar property and function as a heavy metal sorbent in soil. J Agric Food Chem 59:2501–2510
Wang JL, Chen C (2014) Chitosan-based biosorbents: modification and application for biosorption of heavy metals and radionuclides. Bioresour Technol 160:129–141
Xiao X, Chen ZM, Chen BL (2016) H/C atomic ratio as a smart linkage between pyrolytic temperatures, aromatic clusters and sorption properties of biochars derived from diverse precursory materials. Sci Rep-Uk 6:1–13
Xu X, Cao X, Zhao L (2013) Comparison of rice husk- and dairy manure-derived biochars for simultaneously removing heavy metals from aqueous solutions: role of mineral components in biochars. Chemosphere 92:955–961
Xu X, Hu X, Ding Z, Chen Y (2017) Effects of copyrolysis of sludge with calcium carbonate and calcium hydrogen phosphate on chemical stability of carbon and release of toxic elements in the resultant biochars. Chemosphere 189:76–85
Yang HP, Yan R, Chin T, Liang DT, Chen HP, Zheng CG (2004) Thermogravimetric analysis-Fourier transform infrared analysis of palm oil waste pyrolysis. Energ Fuel 18:1814–1821
Yang Y, Wei ZB, Zhang XL, Chen X, Yue DM, Yin Q, Xiao L, Yang LY (2014) Biochar from Alternanthera philoxeroides could remove Pb(II) efficiently. Bioresour Technol 171:227–232
Yuan JH, Xu RK, Zhang H (2011) The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour Technol 102:3488–3497
Yusan S, Gok C, Erenturk S, Aytas S (2012) Adsorptive removal of thorium (IV) using calcined and flux calcined diatomite from Turkey: evaluation of equilibrium, kinetic and thermodynamic data. Appl Clay Sci 67-68:106–116
Funding
The work was supported by the National Natural Science Foundation of China (No. 21677075) and the Project of International Cooperation and Exchange of Nanjing Tech University (2017–2019).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Electronic supplementary material
ESM 1
(DOCX 881 kb)
Rights and permissions
About this article
Cite this article
Chen, T., Zhang, N., Xu, Z. et al. Integrated comparisons of thorium(IV) adsorption onto alkali-treated duckweed biomass and duckweed-derived hydrothermal and pyrolytic biochar. Environ Sci Pollut Res 26, 2523–2530 (2019). https://doi.org/10.1007/s11356-018-3789-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3789-x