Abstract
Pollutants have been proposed as one factor in the worldwide declines of amphibian species and populations. Applying gene expression analysis of liver RNA in tadpoles would be a possible approach for biomarker measurements to increase knowledge of ecological health in amphibian populations. The major aim of this study was to explore the relevance of applying gene expression analyses of cytochrome p450 (cyp1a), metallothionein (mt), and vitellogenin (vtg) in Rana temporaria tadpoles. Therefore, tadpoles were exposed for 1 week to β-naphthoflavone (BNF), cadmium chloride (CdCl2), and ethinylestradiol (EE2). Primers were developed for RT-qPCR to analyze gene expression in livers. The result showed that the methods for gene expression analyses of cyp1a, mt, and vtg as well as the reference gene β-actin (bact) were successful not only in R. temporaria but also in another amphibian, Rana arvalis. The gene expression of cyp1a was induced by BNF and the gene expression of mt was induced by CdCl2 but no significant induction was recorded in vtg expression after exposure to EE2. Gene expressions varied throughout the tadpole metamorphosis development, in particular for vtg. Overall, the use of gene expression of cyp1a and mt as biomarkers in wild tadpoles seems promising while the use of vtg seems less relevant due to high natural variation and low background expression. The study shows that variations in gene expressions between tadpoles of different genetic origin are important to consider when evaluating the data. The present study has thus increased the background knowledge about gene expression applicability as biomarker for tadpoles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pollutants have been proposed as one factor in the worldwide declines of amphibian species and populations (Sparling et al. 2001; Hayes et al. 2010). Pollutants can enter aquatic environments from several sources, such as from pesticide application, which may occur in connection with amphibian reproduction and larval development. Amphibians may be attracted to wetlands such as stormwater ponds, potentially impacted of metals and organic compounds (Simon et al. 2009; Brand and Snodgrass 2010; Pohl et al. 2015) and are thus potentially exposed to a large number of chemicals from different sources during the sensitive development period. Reliable biomarkers that reflects the health and pollutant exposure pressure to amphibians are thus of importance. Biomarkers measured in the tadpole can serve as indicators of pollutant levels and potential health impacts that can be connected to factors in the actual site.
Biomarkers indicating pollution are promising tools to increase knowledge of ecological health and can be applied in effect-based environmental monitoring. There are clear evidence of correlations between different biomarkers and adverse effects such as cytochrome p4501A (CYP1A) activity and dioxin-like toxicity (Whyte et al. 2000) as well as vitellogenin (VTG) levels and effects on sexual development (Örn et al. 2003). However, in many aspects, the use of biomarkers has not yet been fully utilized and integrated in monitoring system (Hook et al. 2014). Further development of tools that can screen multiple responses in parallel and monitor harmful effects of pollutants on ecosystem health are further requested (Baker et al. 2014). Examples of established biomarkers used both in experimental studies and environmental monitoring for identification of exposure to environmental pollutants are CYP1A, VTG, and metallothionein (MT), which indicates exposures to organic contaminants such as polyaromatic hydrocarbons, polychlorinated biphenyls and dioxins, and estrogenic compounds and metals, respectively (Hook et al. 2014). The levels of CYP1A, VTG, and MT have traditionally been determined mainly through enzymatic assays and protein quantification, but also through gene expression, i.e., transcript abundance (Sturve et al. 2005; Lehtonen et al. 2006, Fu et al. 2017). The use of mRNA-based measurement of biomarkers instead of protein measurement provides benefits in efficient utilization of small tissue amount and simplified sampling procedure for field conditions (Quirós et al. 2007). Further, several biomarkers can be measured at individual level within the same isolated RNA-sample (Quirós et al. 2007; Baker et al. 2014).
Rana temporaria (common frog) and Rana arvalis (moor frog) are two relatively prevalent amphibian species in Sweden and northern Europe. We have the last years been studying and sampling tadpoles of these species for different purposes and found them suitable for the use in environmental monitoring. Tadpoles are usually recorded in relatively large numbers and a minor sampling will likely not have a significant impact on future population size, but can add substantially to the knowledge of the general amphibian health. Data obtained from the two Rana species might serve as information also for other more endangered species. Applying gene expression analysis of liver RNA would be a possible approach to get biomarker measurements from individual tadpoles even though liver weights only are a few milligram. The aim of the present study was to establish methods for gene expression analyses of cyp1a, mt, and vtg in livers of R. temporaria tadpoles and to investigate the relevance of applying these as biomarkers indicating chemical pollution on wild tadpoles. We hypothesized that exposure to β-naphthoflavone (BNF), cadmium chloride (CdCl2), and ethinylestradiol (EE2) would increase the hepatic gene expression of cyp1a, mt, and vtg, respectively, in tadpoles of R. temporaria.
Material and methods
Egg collection and tadpole growth
R. temporaria eggs were collected from wild spawnings at three different sites outside Uppsala, Sweden. The distance between the sites (named RT2, RTX, and RT3) were between 5.3 and 23.8 km ensuring genetic diversity between eggs from different sites. Eggs from each site were likely siblings since they were from single clutches. Eggs were raised in site-separated groups in 10 L aquaria with carbon-filtered tap water in room temperature (20 °C). To ensure that sampled eggs were of the correct species, species determination was performed of two individuals from each egg clutch after hatching, according to Palo and Merilä (2003) with slight modifications. Hatched tadpoles were provided duckweed (Lemna minor) and Sera micron powder as feed and 80% water exchange was performed twice a week. When the majority of tadpoles from each site had reached stage 35–36 (Gosner 1960) the exposure started, which means that exposure started at different days for tadpoles from different sites.
Exposure test
One exposure unit consisted of five tadpoles from the same site in 1 L of exposure solution in glass beakers. Exposure solutions consisted of either controls 0.01% DMSO (0.01% DMSO), BNF (300 μg/L), EE2 (0.020 μg/L), or CdCl2 (100 μg/L). In addition, one higher concentration of CdCl2 was included at exposure start for site RT2 (1000 μg/L). This group was however interrupted after 1 day due to high toxicity and replaced with a group of 10 μg/L CdCl2 also used for tadpoles from the other sites. Data obtained from this exposure group were excluded from the study except for the use as reference sample. Stock solutions for all chemicals were prepared in DMSO resulting in 0.01% DMSO in all groups. In total, 60 tadpoles were exposed, i.e., four treatments, tadpoles from three different sites and five tadpoles in each beaker. Exposure solution were totally renewed three times a week (Monday, Wednesday, and Friday) which kept ammonia levels at 4.4 ± 2.5 mg/L before solution change. Temperature were 19.8 ± 0.2 °C throughout the exposure which lasted 7 days and under this period tadpoles were only fed Sera micron.
Sampling
Tadpoles were euthanized in 0.5 g/L MS-222 buffered with NaHCO3. Each tadpole were blotted dry and weighed (BW) to the nearest milligram and measured using a ruler under a stereo microscope to the nearest 0.5 mm. Body length excluding tail (BL), total length including tail (TL), and hind limb length (HLL) was measured as well as determination of the developmental stage. Livers were dissected and stored in Eppendorf tubes with 0.2 mL RNAlater® (Ambion, Inc), the first 24 h in + 4 °C, later in − 18 °C, according to the manufacturers recommendations.
Normal development test
The remaining tadpoles not included in the exposure, continued to grow in the original aquaria. To obtain data reflecting the normal development period throughout the metamorphosis process, one tadpole from each site at each developmental stage from stage 34 up to stage 44, were sampled in the same way as the exposed tadpoles.
RNA isolation, integrity, and concentration determination
RNA was isolated from individual liver samples using NucleoSpin® RNA kit (Macherey-Nagel) according to standard protocol. The RNA concentration and integrity of the samples were measured with Agilent 2100 Bioanalyzer (Agilent Technologies, USA). The RNA samples was later diluted with RNAse free water to obtain a concentration of 10 ng/μL and stored in − 80 °C. As reference sample, a mixture of RNA from five individual livers from the RT3 site exposed to 0.01 μg/L of CdCl2, each contributing with the same amount of RNA, was prepared, aliquoted, and stored in − 80 °C also for future use.
Primer development for R. temporaria and R. arvalis
Primer3web (primer3.ut.ee) was used for primer design. The aim was to get product sizes between 100 and 200 bp for optimal qPCR analyses. Primer selection for β-Actin (bact; reference gene) and Metallothionein (mt) was performed with Multiple Sequence Alignment (Clustal Omega) of published sequences for other amphibian species using the NCBI data base to search for conserved regions, thus likely applicable on R. temporaria and R. arvalis. Published sequences that were included in the alignments were from the following species (including GenBank numbers): Pelophylax esculentus (AY973248.1; HE681912.1), Nanorana parkeri (XM_018571725.1; XM_018555397.1), Bufo gargarizans (EU661596.1), and Rana catesbeiana (GQ222412.1). Primers for Cytochrome p450 1A (cyp1a) were designed from a Xenopus tropicalis sequence (NM_001097344), and then redesigned based on sequenced data of the resulting PCR-product. Vitellogenin (vtg) primers were designed directly from an already existing R. temporaria sequence (JX997958.1). Primers were ordered and synthesized by Eurofins Genomics (Table 1).
Isolated RNA from livers from one R. temporaria and one R. arvalis tadpole collected earlier from wild populations and stored at − 18 °C in RNAlater were used as template for primer development. qRT-PCR (protocol, see section 2.7) was performed using the designed primers including non-template controls (NTCs). Melt curve analyses were performed following each reaction to ensure specific product amplification and check for primer-dimer formation. The resulting PCR-products were sequenced by Macrogen and the identity of each sequence was confirmed by NCBI Basic Local Alignment Search Tool (BLAST) to ensure product specificity. Primer efficiency was confirmed by a fourfold dilution series of RNA for each gene which was run in the real-time PCR, where quantification cycle (Ct) number was plotted against logarithm RNA input. For each gene, an appropriate real-time PCR efficiency (E) was confirmed by calculating the equation E = 10[− 1/slope].
qRT-PCR
Samples (50 ng template RNA per reaction) were run in duplicates in 96-well reaction plates based on the protocol recommended by the manufacturer (QuantiTect® SYBR®Green one-step RT-PCR kit). Deviations from the standard protocol were that a total reaction volume of 25 μL was used instead of 50 μL and that for vtg, primer concentration was reduced to half of the recommended. The process was run on a CFX96™ Real-Time PCR Detection System (Bio Rad). The qPCR protocol consisted of reverse transcription 50 °C (30 min), initial denaturation 95 °C (15 min), and 37 cycles of alternating 94 °C (15 s), 55 °C (30 s), and 72 °C (30 s). The protocol ended with determination of melt curves for each PCR-product. All samples were divided in three separate plates for each primer pair. NTCs and reference samples were included in each plate. Mean of the two duplicates were used for further calculations. Gene expression data of reference gene bact were calculated according to 2-ΔCt (Livak and Schmittgen 2001) where the result of reference sample were used as calibrator. Expression of target genes (cyp1a, mt and vtg) were calculated as 2-ΔΔCt (Livak and Schmittgen 2001).
Statistics
Tadpoles from each site separately were exposed in groups of five in each exposure unit. Despite this, every individual was considered as a replicate further in the data analysis due to practical reasons and that interactions between individuals were not considered likely to affect the result in gene expression. Also, variations in data were suspected to be higher in tadpoles from different sites than in tadpoles from the same site. The different response data in the exposure test were analyzed using general linear models (GLM) with treatment and site as factors including the interaction treatment*site. Homoscedasticity of the data was tested by Levene’s test and if required, data was transformed before the GLM. Dunnett’s post hoc test was used to identify treatment effects significantly different to controls. Tukey’s post hoc test was used to compare differences between sites. Spearman’s nonparametric correlation analysis was used to test relationships between developmental stages and gene expressions. Threshold values for determination of induced expressions were applied for the different genes for the identification of data deviating from normality. These were set according to three times the standard deviation above the control mean. The analyses were made in Minitab® 17.1.0 and the level of significance was set to p < 0.05.
Results
General growth, exposure test
All tadpoles in the highest concentration of CdCl2 (1000 μg/L) which was only started with tadpoles from site RT2 were clearly affected after 1 day of exposure. They were alive, but floating on the surface. Therefore, tadpoles in this group was immediately euthanized and excluded further in the study. One individual exposed to 100 μg/L CdCl2 (site RTX) died by accident in connection to water exchange, so this mortality was not regarded as caused by exposure. No other mortality or other apparently toxic effect was recorded among the remaining tadpoles. There were no effects in growth and development responses (BW, BL, TL, HLL, and Stage) in the exposure test between the different treatment groups (p ≥ 0.154). However, all these responses except BL (p = 0.825) were significantly different between tadpoles from different sites (p ≤ 0.018, Fig. 1). Tadpoles from site RT2 had significantly higher BW than tadpoles from RTX (p < 0.05) and tadpoles from site RT3 had shorter TL then tadpoles from the other sites (p < 0.01). RTX tadpoles were significantly more developed than tadpoles from the other sites, reflected by higher developmental stage (p < 0.001) and longer hind limbs (p < 0.01). No interaction effects between treatment and site were observed among the growth and development responses (p ≥ 0.250).
Growth and development parameters in individual Rana temporaria tadpoles exposed to BNF, CdCl2, EE2, and controls. Tadpoles originate from three different sites (RT2, RTX, and RT3). Graphs show mean ± 95% confidence intervals of body weight (BW, a), body length (BL, b), hind limb length (HLL, c), and a boxplot of developmental stages (d)
Gene expression, exposure test
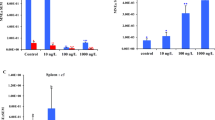
The relative expression between the different genes in the present study were in order mt > bact > cyp1a > > vtg, where vtg were close to the detection limit. Some individuals had vtg expressions below detection limit and were thus excluded from the vtg data, as lack of products were indicated by observations of the melt curves. The gene expression patterns for bact, cyp1a, vtg, and mt in the exposure test are shown in Fig. 2. For all gene expression data, including the reference gene bact, there were differences between sites (p ≤ 0.002). Tadpoles from site RT2 had significantly higher relative expression of bact than tadpoles from the other sites (p < 0.01). Tadpoles from all sites differed regarding relative cyp1a expression (p < 0.001), with highest expression in site RT3 and lowest in RT2. mt expression were significantly higher in site RTX (p < 0.001) while vtg expression were significantly lower in RT2 (p < 0.05) compared with the other sites, respectively.
Hepatic gene expressions relative to reference of bact (a), cyp1a (b), vtg (c), and mt (d) in individual Rana temporaria tadpoles exposed to BNF, CdCl2, EE2, or controls. Tadpoles originate from three different sites (RT2, RTX, and RT3). *** represents significant differences of exposure treatments as compared to controls (p < 0.001; Dunnett’s test). Also, threshold lines are inserted for identification of samples with induced expressions, representing control mean + 3 standard deviations
No significantly different expression of bact was recorded between treatments (p = 0.479) and neither any interaction effect between treatment and site (p = 0.263). cyp1a expression was affected by treatment (p = 0.000). The tadpoles exposed to BNF had a higher expression compared with the controls (p < 0.001). Also, there was an interaction effect in cyp1a expression between treatment and site (p = 0.000). There were no treatment effect recorded on vtg expression (p = 0.143) and neither any interaction effect between treatment and site (p = 0.354). Expression of mt was affected by treatment (p = 0.000). The tadpoles exposed to CdCl2 had a higher expression compared with the controls (p < 0.001). Also, there was an interaction effect in cyp1a expression between treatment and site (p = 0.002).
A positive correlation between stage and bact expression was recorded (Spearman’s rho (ρ) = 0.367; p = 0.046) when including all data in the exposure test. The correlation analyses also revealed some associations between gene expressions expected to be upregulated by chemical exposure and the developmental stage. Correlation analysis between stage and cyp1a expression in the tadpoles exposed to BNF also showed a positive correlation (ρ = 0.527; p = 0.044). Further, vtg showed a positive correlation with stage in EE2-exposed tadpoles (ρ = 0.659; p = 0.008). mt expression was however not correlated with stage among CdCl2-exposed tadpoles (ρ = 0.520, p = 0.057). Threshold values for determination of induced relative expressions were determined to be 1.38, 1.46, and 2.98 for cyp1a, vtg, and mt, respectively (Fig. 2).
Normal development test
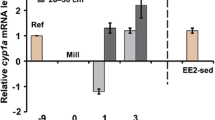
The growth and gene expression data throughout the metamorphosis period between stages 34 and 44 are shown in Fig. 3. Correlation analyses between stage and the different gene expressions revealed positive correlations for bact (ρ = 0.367; p = 0.046) and vtg (ρ = 0.675; p = 0,000), but no correlations for cyp1a (ρ = 0.211; p = 0.262) or mt (ρ = 0.025; p = 0.897).
Discussion
One aim of the present study was to establish methods for gene expression analyses of cyp1a, mt, and vtg in livers of R. temporaria tadpoles. This aim was fulfilled since the designed primers including primers for the reference gene bact were able to bind to hepatic RNA and amplify expected sequences with the used PCR-protocol, as indicated by runs of the sequenced PCR-products in BLAST. We have also shown that these primers work with the same protocol in another relative species, R. arvalis.
Another aim was to investigate the relevance of applying these genes as biomarkers indicating chemical pollution on wild tadpoles. This needs to be considered by several aspects in order to provide a proper assessment. When studying biomarker effects in wild populations, it is fundamental to be aware of how natural variation affects the different measured responses. To include some degree of genetic intraspecific variation, R. temporaria eggs were collected from three different sites and were kept separate throughout the whole study. The results from most recorded responses in the exposure test revealed significant variations between tadpoles from different sites. This reflects different growth rates, development progress, and expression of the various biomarkers, which is important to be aware of when applying biomarkers in environmental monitoring programs. Otherwise, there is a risk that a potential difference in gene expression caused by genetic variation is misinterpreted as effects due to chemical exposure. Also, expression of the reference gene (bact) showed clear significant differences between sites which also might have influenced the variation between sites in the expressions of other genes. The expression profile of bact in unexposed tadpoles throughout the metamorphosis is somewhat inconsistent (Fig. 3c). A positive correlation between stage and bact expression was recorded but there are also sudden low expressions at stages 41 and 44, which might imply that bact is not a perfect choice as reference gene. Developmental changes in the expression of reference genes during amphibian development have previously been recorded (Hogan et al. 2007; Navarro-Martín et al. 2012). However, bact expression was not influenced by the different chemical exposures, which much is considered as the most important condition for a reference gene that is used in connection to biomarker genes for specific chemical impact.
We aimed for exposing the tadpoles to concentrations of chemicals that did not cause any overt toxicity to but high enough to cause inductions in gene expressions. The concentrations of different chemicals were thus not chosen as pollutants in relevant concentrations but rather as model chemicals suspected to impact the gene expressions of interest. A BNF concentration of 300 μg/L was based on a pilot study where we observed a fourfold increase in CYP1A activity (measured using ethoxyresorufin-O-deethylase) on tadpole livers of R. temporaria exposed for 1 week to 300 μg/L BNF, but no induction at 30 μg/L (unpublished data). The EE2 concentration was based on literature on data from protein and gene expression analyses on zebrafish and X. tropicalis frogs (Örn et al. 2003; Säfholm et al. 2015). We included two starting concentrations of CdCl2 (100 and 1000 μg/L) due to varying and limiting toxicity information. The highest concentration was replaced with a lower (10 μg/L) due to toxicity but since there was no toxicity recorded in the 100 μg/L, no analyses were performed in the lower concentration.
As expected, the gene expressions of cyp1a were significantly upregulated in tadpoles exposed to BNF in comparison to the controls. However, this upregulation was not as clear in tadpoles from site RT2 as from the other sites (Fig. 2b). The developmental stages for tadpoles from site RT2 were relatively low and tadpoles from site RTX, which had the highest expression, were in slightly higher developmental stages (Fig. 1d). A positive correlation between cyp1a expression and stage among the BNF-exposed tadpoles was detected, which might indicate that tadpoles are not fully responding to BNF exposure until later developmental stages. The expressions of cyp1a in unexposed tadpoles from stage 34 to 44 showed a non-monotonic pattern with slightly higher expressions between stages 37 and 40 but lower before and after these stages. No clear correlation between stage and cyp1a expression was detected among these tadpoles.
We did not reveal any significant vtg expression increase in tadpoles exposed to EE2 even though some clearly higher values were obtained in the group from site RTX (Fig. 2c). These tadpoles were the most developed among EE2-exposed tadpoles (stages 40 and 41) and the correlation between stage and vtg expression among EE2-exposed tadpoles indicates that tadpoles in later stages are much more responsive to EE2 exposure than in lower stages. Unexposed tadpoles in stages 42 and higher have an even higher expression of vtg than the EE2-exposed (Fig. 3e). These findings complicate the development of vtg to be used as a biomarker based on the present study. Only the later tadpole stages appears to be sensitive to vtg expression and it requires detailed studies to be able to separate between natural and induced variations in vtg expression. The vtg expressions were also close to the detection limit at lower stages. It has been shown that synthetic estrogens can induce vtg expression in newly metamorphosed amphibians (Säfholm et al. 2015). However, studies of vtg expression at lower tadpole stages are scarce. The use of VTG as biomarker for estrogenic activity is generally based on males or juveniles, in which the endogenous VTG production is low, but can respond to estrogenic exposure (Wheeler et al. 2005). The increase in vtg expression in later developmental stages observed in the present study is probably due to gonad development in females. It has been reported that differentiation of ovaries has its own timing, independent of somatic development in R. temporaria and other amphibians (Ogielska and Kotusz 2004). We can thus expect that vtg expression do not follow the developmental process and thereby complicates the data interpretation further.
The gene expressions of mt were significantly upregulated in tadpoles exposed to CdCl2 in comparison to the controls (Fig. 2d). However, much of the data of the tadpoles expected to have induced expressions due to CdCl2 exposure are “hidden” within the normal variation of tadpoles from different sites. The expression seems not to be correlated to the developmental stage neither among CdCl2 exposed nor in unexposed tadpoles. It is possible that a higher concentration of CdCl2 would have revealed a more pronounced induction of mt, but the ten-time higher concentration used in the start of the experiment caused clear toxicity which makes it unrealistic for the use as positive control for biomarker development.
The reason for evaluating the method for gene expression analysis in the present study was to apply gene expression data as biomarkers for chemical exposure in wild tadpoles in future studies. As a diagnostic tool, threshold values for determination of induced expressions were applied for the different genes for the identification of data deviating from normality. These thresholds seem to be reasonable in terms of avoiding detecting false positive based on the data in the present study (Fig. 2). However, many individuals that belonged to groups, which were hypothesized to have increased expressions of specific genes, were recorded as negative. Only three data points among CdCl2-exposed tadpoles were above the threshold value for mt and five data points among EE2-exposed tadpoles were above the threshold value for vtg. For tadpoles exposed to BNF, most expressions of cyp1a were above the threshold value and thus detected as positive, but also here, all BNF-exposed tadpoles from site RT2 were recorded as negative. These threshold values might need to be further verified by the use in real biomonitoring studies, in particular where chemical analyses can provide data for correlations between effect and exposure. Overall, the use of cyp1a as biomarker on R. temporaria tadpoles seems to be promising. Wild tadpoles can potentially be exposed to many pollutants causing increases in gene expression of cyp1a since other pollutants have been shown to trigger CYP1A activity or gene expression with different potencies than BNF (Smeets et al. 1999). mt might be somewhat useful for biomonitoring even though the present study indicates that most individuals exposed to CdCl2 were not distinguished from the background. In the present study, mt induction was triggered by exposure to CdCl2 but there might be other metals or higher metal concentrations that are more potent as mt inducers. In carps (Cyprinus carpio), mercury and silver exposure was recorded to induce liver mt more as compared with cadmium (Cosson 1993). A similar pattern was observed in 32-h-old zebrafish embryos where mercury were more potent than cadmium, which in turn was more potent than zinc and copper in induction of mt gene expression (Chan et al. 2006). There might be influences on mt expression also due to metal uptake. All chemicals were exposed via the surrounding water in the present study. It is more likely that organic pollutants but also metals are particle bound or accumulated in plants in wetlands and are thus exposed to tadpoles through feed or by sediment contact which might give other uptake and response patterns. Tadpoles in the present study were exposed for 1 week before gene expression analyses. In natural habitats where tadpoles have been developed and thereby also exposed from their embryo stages, this might also result in different gene expression responses as compared with the present study. The use of vtg as biomarker for estrogenic exposure in tadpoles is less promising based on the present study. There are several indications suggesting that the use of vtg as biomarker on tadpoles is questionable, due to low gene expression and high natural variation, thereby complicating the detection of affected individuals.
We have shown that the designed primers also work for the use in R. arvalis. However, we do not have the background information regarding induction patterns from the positive control substances. To be able to identify deviations from normal expressions also in R. arvalis, we might need to perform corresponding exposure studies. Another approach is to hypothesize that both species respond equal to environmental conditions. In samplings from wild populations, tadpoles from both species are often caught from the same ponds and thus correlations in gene expression data between both species can be made to verify or reject this hypothesis.
Conclusions
We have established methods for gene expression analyses of cyp1a, mt, and vtg in livers of Rana temporaria and Rana arvalis tadpoles. The hepatic gene expression of cyp1a was induced by BNF and the gene expression of mt was induced by CdCl2 when R. temporaria tadpoles were exposed for 1 week to the different chemicals. There was no significant induction in hepatic vtg expression in R. temporaria tadpoles after exposure to EE2. The use of cyp1a and mt as biomarkers in wild tadpoles for pollution of organic compounds and metals, respectively, seems promising. vtg gene expression data seem less promising as biomarker for estrogenic activity due to high natural variation. The present study shows that variations in gene expressions are high between tadpoles of different genetic origin and that this must be considered in the interpretation of data. By this study, more knowledge is obtained about gene expression applicability as biomarker in tadpoles of Rana temporaria for monitoring of pollutant exposure.
References
Baker ME, Sprague LJ, Ribecco C, Ruggeri B, Lekmine N, Ludka C, Wick I, Soverchia L, Ubaldi M, Šášik R, Schlenk D, Kelley KM, Reyes JA, Hardiman G (2014) Application of a targeted endocrine q-PCR panel to monitor the effects of pollution in southern California flatfish. Endocr Disruptors 2(1):e969598
Brand AB, Snodgrass JW (2010) Value of artificial habitats for amphibian reproduction in altered landscapes. Conserv Biol 24:295–301
Chan KM, Ku LL, Chan PC, Cheuk WK (2006) Metallothionein gene expression in zebrafish embryo-larvae and ZFL cell-line exposed to heavy metal ions. Mar Environ Res 62:S83–S87
Cosson RP (1993) Heavy metal intracellular balance and relationship with metallothionein induction in the liver of carp after contamination by silver, cadmium and mercury following or not pretreatment by zinc. BioMetals 7:9–19
Fu D, Bridle A, Leef M, Gagnon MM, Hassell KL, Nowak BF (2017) Using a multi-biomarker approach to assess the effects of pollution on sand flathead (Platycephalus bassensis) from Port Phillip Bay, Victoria, Australia. Mar Pollut Bull 119:211–219
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Hayes TB, Falso P, Gallipeau S, Stice M (2010) The cause of global amphibian declines: a developmental endocrinologist's perspective. J Exp Biol 213:921–933
Hogan NS, Crump KL, Duarte P, Lean DRS, Trudeau VL (2007) Hormone cross-regulation in the tadpole brain: developmental expression profiles and effect of T3 exposure on thyroid hormone- and estrogen-responsive genes in Rana pipiens. Gen Comp Endocrinol 154:5–15
Hook SE, Gallagher EP, Batley GE (2014) The role of biomarkers in the assessment of aquatic ecosystem health. Integr Environ Assess Manag 10:327–341
Lehtonen KK, Schiedek D, Köhler A, Lang T, Vuorinen PJ, Förlin L, Barsiene J, Pempkowiak J, Gercken J (2006) The BEEP project in the Baltic Sea: overview of results and outline for a regional biological effects monitoring strategy. Mar Pollut Bull 53:523–537
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–408
Navarro-Martín L, Lanctôt C, Edge C, Houlahan J, Trudeau VL (2012) Expression profiles of metamorphosis-related genes during natural transformations in tadpoles of wild wood frogs (Lithobates sylvaticus). Can J Zool 90:1059–1071
Ogielska M, Kotusz A (2004) Pattern and rate of ovary differentiation with reference to somatic development in anuran amphibians. J Morphol 259:41–54
Örn S, Holbech H, Madsen TH, Norrgren L, Petersen GI (2003) Gonad development and vitellogenin production in zebrafish (Danio rerio) exposed to ethinylestradiol and methyltestosterone. Aquat Toxicol 65:397–411
Palo JU, Merilä J (2003) A simple RFLP method for identification of two ranid frogs. Conserv Genet 4:801–803
Pohl J, Örn S, Norrgren L, Carlsson G (2015) Toxicological evaluation of water from stormwater ponds using Xenopus tropicalis embryos. Wetl Ecol Manag 23:1091–1098
Quirós L, Piña B, Solé M, Blasco J, López MA, Riva MC, Barceló D, Raldúa D (2007) Environmental monitoring by gene expression biomarkers in Barbus graellsii: laboratory and field studies. Chemosphere 67:1144–1154
Säfholm M, Jansson E, Fick J, Berg C (2015) Mixture effects of levonorgestrel and ethinylestradiol: estrogenic biomarkers and hormone receptor mRNA expression during sexual programming. Aquat Toxicol 161:146–153
Simon JA, Snodgrass JW, Casey RE, Sparling DW (2009) Spatial correlates of amphibian use of constructed wetlands in an urban landscape. Landsc Ecol 24:361–373
Smeets JM, van Holsteijn I, Giesy JP, van den Berg M (1999) The anti-estrogenicity of Ah receptor agonists in carp (Cyprinus carpio) hepatocytes. Toxicol Sci 52:178–188
Sparling DW, Fellers GM, McConnell LL (2001) Pesticides and amphibian population declines in California, USA. Environ Toxicol Chem 20(7):1591–1595
Sturve J, Berglund A, Balk L, Broeg K, Böhmert B, Massey S, Savva D, Parkkonen J, Stephensen E, Koehler A, Förlin L (2005) Effects of dredging in Göteborg harbor, Sweden, assessed by biomarkers in eelpout (Zoarces viviparus). Environ Toxicol Chem 24:1951–1961
Wheeler JR, Gimeno S, Crane M, Lopez-Juez E, Morritt D (2005) Vitellogenin: a review of analytical methods to detect (anti) estrogenic activity in fish. Toxicol Mech Methods 15:293–306
Whyte JJ, Jung RE, Schmitt CJ, Tillitt DE (2000) Ethoxyresorufin-O-deethylase (EROD) activity in fish as a biomarker of chemical exposure. Crit Rev Toxicol 30:347–570
Acknowledgments
The study was a part of the project “Tadpoles as bioindicators” financially supported within the Environmental monitoring and assessment program at the Swedish University of Agricultural Sciences. We are also grateful to Moa Skarin for excellent laboratory work and to Jari Parkkonen at the University of Gothenburg for Cyp1A activity analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the ethical committee for animal research (5.8.18-03551/2017). Approval for collection of R. temporaria eggs was obtained from the county administration in Uppsala, Sweden (522-1086-16).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Carlsson, G., Tydén, E. Development and evaluation of gene expression biomarkers for chemical pollution in common frog (Rana temporaria) tadpoles. Environ Sci Pollut Res 25, 33131–33139 (2018). https://doi.org/10.1007/s11356-018-3260-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3260-z





 , RTX
, RTX  , and RT3
, and RT3 ), sampled at different stages throughout the metamorphosis
), sampled at different stages throughout the metamorphosis