Abstract
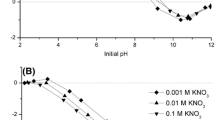
Siderophores, as strong chelators specific to iron, have been intensively studied in relation to the facilitation of biological iron acquisition from iron oxides. In this study, the dissolution of hematite in the presence of the siderophore desferrioxamine B (DFOB) and three low-molecular-weight organic acids (LMWOAs, i.e., oxalic, citric, or malic acid) was investigated at three pH conditions (3.0, 5.5, and 9.0). Hematite dissolution was pH-dependent and LMWOA-specific. The adsorption of DFOB on hematite was significantly higher at pH 9.0 than at the other pH values. The adsorption of oxalic acid on hematite, however, showed a descending trend as pH was increased, and adsorption of citric and malic acids was not significantly affected by pH. The Fourier transform infrared (FTIR) results also indicated the occurrence of these ligands’ adsorption. After acidification, dissolved iron was detected only in suspensions of hematite pre-adsorbed with oxalic acid at pH 5.5 and 9.0 or pre-adsorbed with citric acid at pH 5.5, indicating that these LMWOAs promoted the formation of labile iron on the hematite surface. Based on previous research and the results of this study, a hypothetical model is proposed. These results provide insight into the effect of LMWOAs on the dissolution of hematite promoted by DFOB.






Similar content being viewed by others
References
Bai J, Yang X, Du R, Chen Y, Wang S, Qiu R (2014) Biosorption mechanisms involved in immobilization of soil Pb by Bacillus subtilis DBM in a multi-metal-contaminated soil. J Environ Sci-China 26:2056–2064
Bray AW, Oelkers EH, Bonneville S, Wolff-Boenisch D, Potts NJ, Fones G, Benning LG (2015) The effect of pH, grain size, and organic ligands on biotite weathering rates. Geochim Cosmochim Acta 164:127–145
Cervini-Silva J, Sposito G (2002) Steady-state dissolution kinetics of aluminum-goethite in the presence of desferrioxamine-B and oxalate ligands. Environ Sci Technol 36:337–342
Cheah S-F, Kraemer SM, Cervini-Silva J, Sposito G (2003) Steady-state dissolution kinetics of goethite in the presence of desferrioxamine B and oxalate ligands: implications for the microbial acquisition of iron. Chem Geol 198:63–75
Cocozza C, Tsao CCG, Cheah S-F, Kraemer SM, Raymond KN, Miano TM, Sposito G (2002) Temperature dependence of goethite dissolution promoted by trihydroxamate siderophores. Geochim Cosmochim Acta 66:431–438
Colombo C, Palumbo G, He J-Z, Pinton R, Cesco S (2014) Review on iron availability in soil: interaction of Fe minerals, plants, and microbes. J Soils Sed 14:538–548
Dehner CA, Awaya JD, Maurice PA, DuBois JL (2010) Roles of Siderophores, oxalate, and ascorbate in mobilization of iron from hematite by the aerobic bacterium Pseudomonas mendocina. Appl Environ Microbiol 76:2041–2048
D'Onofrio A, Crawford JM, Stewart EJ, Witt K, Gavrish E, Epstein S, Clardy J, Lewis K (2010) Siderophores from neighboring organisms promote the growth of uncultured bacteria. Chem Biol 17:254–264
Duckworth OW, Martin ST (2001) Surface complexation and dissolution of hematite by C1-C6 dicarboxylic acids at pH = 5.0. Geochim. Cosmochim. Acta 65:4289–4301
Furrer G, Stumm W (1986) The coordination chemistry of weathering: I. Dissolution kinetics of δ- Al2O3 and BeO. Geochim. Cosmochim. Acta 50:1847–1860
Glick BR (2010) Using soil bacteria to facilitate phytoremediation. Biotechnol Adv 28:367–374
Haack EA, Johnston CT, Maurice PA (2008) Mechanisms of siderophore sorption to smectite and siderophore-enhanced release of structural Fe3+. Geochim Cosmochim Acta 72:3381–3397
Jones DL (1998) Organic acids in the rhizosphere–a critical review. Plant Soil 205:25–44
Jones DL, Darah PR, Kochian LV (1996) Critical evaluation of organic acid mediated iron dissolution in the rhizosphere and its potential role in root iron uptake. Plant Soil 180:57–66
Jones DL, Dennis PG, Owen AG, van Hees PAW (2003) Organic acid behavior in soils—misconceptions and knowledge gaps. Plant Soil 248:31–41
Kraemer SM (2004) Iron oxide dissolution and solubility in the presence of siderophores. Aquat Sci 66:3–18
Kraemer SM, Cheah SF, Zapf R, Xu J, Raymond KN, Sposito G (1999) Effect of hydroxamate siderophores on Fe release and Pb (II) adsorption by goethite. Geochim Cosmochim Acta 63:3003–3008
Noerpel MR, Lenhart JJ (2015) The impact of particle size on the adsorption of citrate to hematite. J Colloid Interface Sci 460:36–46
Parker DR, Chaney RL, Norvell WA (1995) Chemical equilibrium models: applications to plant nutrition research. In: Loeppert RH, Schwab AP, Goldberg S (eds) Chemical equilibrium and reaction models. SSSA Special Publication. Soil Science Society of America and American Society of Agronomy, Madison, pp 163–200
Rajkumar M, Ae N, Prasad MNV, Freitas H (2010) Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol 28:142–149
Reichard PU, Kraemer SM, Frazier SW, Kretzschmar R (2005) Goethite dissolution in the presence of Phytosiderophores: rates, mechanisms, and the synergistic effect of oxalate. Plant Soil 276:115–132
Reichard PU, Kretzschmar R, Kraemer SM (2007) Dissolution mechanisms of goethite in the presence of siderophores and organic acids. Geochim Cosmochim Acta 71:5635–5650
Saha M, Sarkar S, Sarkar B, Sharma BK, Bhattacharjee S, Tribedi P (2016) Microbial siderophores and their potential applications: a review. Environ Sci Pollut R 23:3984–3999
Samson SD, Eggleston CM (2002) Nonsteady-state dissolution of goethite and hematite in response to pH jumps: the role of adsorbed Fe (III). In: Hellmann R, Wood SA (Eds), Water-Rock Interactions, Ore Deposits, and Environmental Geochemistry: A Tribute to David A. Crerar. The Geochemical Society, pp. 61–73
Simanova AA, Persson P, Loring JS (2010) Evidence for ligand hydrolysis and Fe(III) reduction in the dissolution of goethite by desferrioxamine-B. Geochim Cosmochim Acta 74:6706–6720
Smith RM, Martell AE, Motekaitis RJ (2004) NIST critically selected stability constants of metal complexes, NIST Standard Reference Database 46. http://www.nist.gov/srd/upload/46_8.pdf. Accessed 6 July 2016
Son J-S, Sumayo M, Hwang Y-J, Kim B-S, Ghim S-Y (2014) Screening of plant growth-promoting rhizobacteria as elicitor of systemic resistance against gray leaf spot disease in pepper. Appl Soil Ecol 73:1–8
Sugimoto T, Sakat K, Muramatsu A (1992) Formation mechanism of monodisperse pseudocubic α-Fe2O3 particles from condensed ferric hydroxide gel. J Colloid Interface Sci 159:587–590
Tian S-K, Lu L-L, Yang X-E, Huang H-G, Brown P, Labavitch J, Liao H-B, He Z-L (2011) The impact of EDTA on lead distribution and speciation in the accumulator Sedum alfredii by synchrotron X-ray investigation. Environ Pollut 159:782–788
Tomasi N, Mimmo T, Terzano R, Alfeld M, Janssens K, Zanin L, Pinton R, Varanini Z, Cesco S (2014) Nutrient accumulation in leaves of Fe-deficient cucumber plants treated with natural Fe complexes. Biol. Fertility Soils 50:973–982
Wang Q, Xiong D, Zhao P, Yu X, Tu B, Wang G (2011) Effect of applying an arsenic-resistant and plant growth–promoting rhizobacterium to enhance soil arsenic phytoremediation by Populus deltoides LH05-17. J Appl Microbiol 111:1065–1074
Xu N, Gao Y (2008) Characterization of hematite dissolution affected by oxalate coating, kinetics and pH. Appl Geochem 23:783–793
Yang B, Hoegy F, Mislin GLA, Mesini PJ, Schalk IJ (2011) Terbium, a fluorescent probe for investigation of siderophore pyochelin interactions with its outer membrane transporter FptA. J Inorg Biochem 105:1293–1298
Yoshida T, Hayashi K-I, Ohmoto H (2002) Dissolution of iron hydroxides by marine bacterial siderophore. Chem Geol 184:1–9
Acknowledgements
The authors are thankful for the financial support from the National Science Foundation Committee of China (Nos. 41171374,41101483 and 41671313), the Fundamental Research Funds for the Central Universities of China (No. 16lgjc57), the National Science Foundation for Distinguished Young Scholars of China (No. 41225004), Science and Technology Planning Project of Guangdong Province, China (No. 2014A050503032), and the Research Fund Program of Guangdong Provincial Key Laboratory of Environmental Pollution Control and Remediation Technology (2016 K0005).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This study was funded by the National Science Foundation Committee of China (Nos. 41171374, 41101483, and 41671313), the Fundamental Research Funds for the Central Universities of China (No. 16lgjc57), the National Science Foundation for Distinguished Young Scholars of China (No. 41225004), Science and Technology Planning Project of Guangdong Province, China (No. 2014A050503032), and the Research Fund Program of Guangdong Provincial Key Laboratory of Environmental Pollution Control and Remediation Technology (2016K0005).
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/ or animals
This research does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Lin, Q., Wang, Y., Yang, X. et al. Effect of low-molecular-weight organic acids on hematite dissolution promoted by desferrioxamine B. Environ Sci Pollut Res 25, 163–173 (2018). https://doi.org/10.1007/s11356-017-9045-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9045-y