Abstract
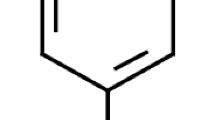
Fulvic acid (Henan ChangSheng Corporation) photoinduced degradation of non-UVA-absorbing herbicide amitrole (3-amino-1,2,4-triazole, AMT) as a way for its removal from polluted water was investigated in details. It was shown that the main primary species generated by fulvic acid under UVA radiation, triplet state and hydrated electron, are not directly involved in the herbicide degradation. AMT decays in reactions with secondary intermediates, reactive oxygen species, formed in reactions of the primary ones with dissolved oxygen. Singlet oxygen is responsible for 80% of herbicide oxidation, and •OH and O2 −• radicals—for the remaining 20% of AMT. It was found that quantum yield of AMT photodegradation (ϕ 365nm) decreases linearly from 2.2 × 10−3 to 1.2 × 10−3 with the increase of fulvic acid concentration from 1.1 to 30 mg L−1. On the contrary, the increase of AMT concentration from 0.8 to 25 mg L−1 leads to practically linear growth of ϕ 365nm value from 1.8 × 10−4 to 4 × 10−3. Thus, the fulvic acid exhibits a good potential as UVA photooxidizer of organic pollutants sensitive to the singlet oxygen (ϕ 532nm(1O2) = 0.025 at pH 6.5).




Similar content being viewed by others
References
Alfassi ZB, Schuler RH (1985) Reaction of azide radicals with aromatic compounds. Azide as a selective oxidant. J Phys Chem 89:3359–3363. doi:10.1021/j100261a040
Bamford D, Sorsa M, Gripenberg U, Laamanen I, Meretoja T (1976) Mutagenicity and toxicity of amitrole. III. Microbial tests. Mutation Res/Gen Toxicol 40:197–202. doi:10.1016/0165-1218(76)90045-8
Blough NV, Zepp RG (1995) Reactive oxygen species in natural waters. In: Foote CS, Valentine JS, Greenberg A, Liebman JF (eds) Active oxygen in chemistry. Chapman and Hall, New York, pp 280–331. doi:10.1007/978-94-007-0874-7_8
Brouwer AM (2011) Standards for photoluminescence quantum yield measurements in solution (IUPAC Technical Report). Pure Appl Chem 83:2213–2228. doi:10.1351/PAC-REP-10-09-31
Buxton GV, Greenstock CL, Helman WP, Ross AB (1988) Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (•OH/•O−) in aqueous solution. J Phys Chem Ref Data 17:513. doi:10.1063/1.555805
Catastini C, Rafqah S, Mailhot G, Sarakha M (2004) Degradation of amitrole by excitation of Fe(III) aquo complexes in aqueous solutions. J Photochem Photobiol A 162:97–103. doi:10.1016/S1010-6030(03)00316-2
Chaikovskaya ON, Levin PP, Sul'timova NB, Sokolova IV, Kuz"min AV (2004) Triplet states of humic acids studied by laser flash photolysis using different excitation wavelengths. Russ Chem B, Int Ed 53:313–317. doi:10.1023/B:RUCB.0000030803.83476.4e
Chen Y, Hu C, Hu X, Qu J (2009) Indirect photodegradation of amine drugs in aqueous solution under simulated sunlight. Environ. Sci. Technol. 43:2760–2765. doi:10.1021/es803325j
Chen Y, Li H, Wang Z, Li H, Tao T, Zuo Y (2012) Photodegradation of selected β-blockers in aqueous fulvic acid solutions: kinetics, mechanism, and product analysis. Water Res 46:2965–2972. doi:10.1016/j.watres.2012.03.025
Cooper, W.J., Zika, R.G., Petasne, R.G., Fischer, A.M. (1989) Sunlight-induced photochemistry of humic substances in natural waters: major reactive species. In: Suffet, I.H., MacCarthy, P. Eds., ACS Symposium Series, vol. 219. American Chemical Society, Washington, DC, pp. 333–362. Doi: 10.1021/ba-1988-0219.ch022
Dalrymple RM, Carfagno AK, Sharpless C (2010) Correlations between dissolved organic matter optical properties and quantum yields of singlet oxygen and hydrogen peroxide. Environ Sci Technol 44:5824–5829. doi:10.1021/es101005u
de la d’Auzay Rochebrochard S, Brosillon S, Fourcade F, Amrane A (2007) Integrated Process for Degradation of Amitrole in Wastewaters: Photocatalysis/Biodegradation. Int. J. Chem. Reactor Eng. 5 Article A51. doi: 10.2202/1542-6580.1453
Fischer AM, Kliger DS, Winterle JS, Mill T (1985) Direct observation of phototransients in natural waters. Chemosphere 14:1299–1306. doi:10.1016/0045-6535(85)90150-X
Fischer AM, Winterle JM, Mill T (1987) Photochemistry of environmental aquatic systems. In: Zika RG, Cooper WJ (eds) ACS Symposium Series 327. American Chemical Society, Chapter 11, Washington, DC, pp 141–156. doi:10.1021/bk-1987-0327.ch011
Frederiksen PK, McIlroy SP, Nielsen CB, Nikolajsen L, Skovsen E, Jörgensen M, Mikkelsen KV, Ogilby PR (2005) Two-photon photosensitized production of singlet oxygen in water. J Am Chem Soc 127:255–269. doi:10.1021/ja0452020
Furukawa A, Oikawa S, Harada K, Sugiyama H, Hiraku Y, Murata M, Shimada A, Kawanishi S (2010) Oxidatively generated DNA damage induced by 3-amino-5-mercapto-1,2,4-triazole, a metabolite of carcinogenic amitrole. Mutat Res 694:7–12. doi:10.1016/j.mrfmmm.2010.08.004
Gaines TB, Kimbrough RD, Linder RE (1973) The toxicity of amitrole in the rat. Toxicol. Appl. Pharm. 26:118–129. doi:10.1016/0041-008X(73)90092-6
Galievsky VA, Stasheuski AS, Kiselyov VV, Shabusov AI, Belkov MV, Dzhagarov BM (2010) Laser NIR lifetime spectrometer with nanosecond time resolution. Instrum Exp Tech 53:568–574. doi:10.1134/S0020441210040184
Gollnick K, Schenck GO (1967) Organic chemistry. In: Hamer J (ed) 1,4-Cycloaddition reactions the diels-alder reaction in heterocyclic syntheses, chapter 10, vol 8. pp 255–344. doi:10.1016/B978-0-12-395743-6.50015-6
Haag WR, Hoigne J, Gassman E, Braun AM (1984) Singlet oxygen in surface waters: part II. Quantum yields of its production by some natural humic materials as a function of wavelength. Chemosphere 13:641–650. doi:10.1016/0045-6535(84)90200-5
Hare PM, Price EA, Bartels DM (2008) Hydrated electron extinction coefficient revisited. J Phys Chem A 112:6800–6802. doi:10.1021/jp804684w
Jensen-Korte U, Anderson C, Spiteller M (1987) Photodegradation of pesticides in the presence of humic substances. Sci Total Environ 62:47–54. doi:10.1016/0048-9697(87)90518-3
Klaus U, Oesterreich T, Volk M, Spiteller M (1998) Interaction of aquatic dissolved organic matter (DOM) with amitrole: the nature of the bound residues. Acta Hydrochim Hydrobiol 26:311–317. doi:10.1002/(SICI)1521-401X(199811)26:6<311::AID-AHEH311>3.0.CO;2-X
Lou T, Xie H (2006) Photochemical alteration of the molecular weight of dissolved organic matter. Chemosphere 65:2333–2342. doi:10.1016/j.chemosphere.2006.05.001
Makunina MP, Pozdnyakov IP, Grivin VP, Plyusnin VF (2014) Investigation of fulvic acid photochemistry in aqueous solutions by stationary and laser flash photolysis techniques. High Energy Chem. 48:197–201. doi:10.1134/S0018143914030072
Makunina MP, Pozdnyakov IP, Chen Y, Grivin VP, Bazhin NM, Plyusnin VF (2015) Mechanistic study of fulvic acid assisted photodegradation of propranolol in aqueous solution. Chemosphere 119:1406–1410. doi:10.1016/j.chemosphere.2014.10.008
McNeill K, Canonica S (2016) Triplet state dissolved organic matter in aquatic photochemistry: reaction mechanisms, substrate scope, and photophysical properties. Environ Sci: Processes Impacts 18:1381–1399. doi:10.1039/c6em00408c
Neta P, Huie RE, Ross AB (1988) Rate constants for reactions of inorganic radicals in aqueous solution. J Phys Chem Ref Data 17:1027. doi:10.1063/1.555808
Orellana-García F, Álvarez MA, López-Ramón V, Rivera-Utrilla J, Sánchez-Polo M, Mota AJ (2014) Photodegradation of herbicides with different chemical natures in aqueous solution by ultraviolet radiation. Effects of operational variables and solution chemistry. Chem Eng J 255:307–315. doi:10.1016/j.cej.2014.06.047
Orellana-García F, Álvarez MA, López-Ramón V, Rivera-Utrilla J, Sánchez-Polo M (2015) Effect of HO•, SO4 •– and CO3 •−/HCO3 • radicals on the photodegradation of the herbicide amitrole by UV radiation in aqueous solution. Chem Eng J 267:182–190. doi:10.1016/j.cej.2015.01.019
Paul A, Hackbarth S, Vogt RD, Burnison BK, Steinberg CEW (2004) Photogeneration of singlet oxygen by humic substances: comparison of humic substances of aquatic and terrestrial origin. Photochem Photobiol Sci 3:273. doi:10.1039/B312146A
Pozdnyakov IP, Plyusnin VF, Grivin VP, Vorobyev DY, Bazhin NM, Vauthey E (2006) Photolysis of sulfosalicylic acid in aqueous solutions over a wide pH range. J Photochem Photobiol A Chem 181:37–43. doi:10.1016/j.jphotochem.2005.10.030
Sánchez-Bayoa F, Hyne RV, Desseille KL (2010) An amperometric method for the detection of amitrole, glyphosate and its aminomethyl-phosphonic acid metabolite in environmental waters using passive samplers. Anal Chim Acta 675:125–131. doi:10.1016/j.aca.2010.07.013
Sharpless CM, Aeschbacher M, Page SE, Wenk J, Sander M, McNeill K (2014) Photooxidation-induced changes in optical, electrochemical, and photochemical properties of humic substances. Environ Sci Technol 48:2688–2696. doi:10.1021/es403925g
Stasheuski AS, Galievsky VA, Knyukshto VN, Ghazaryan RK, Gyulkhandanyan AG, Gyulkhandanyan GV, Dzhagarov BM (2014) Water-soluble pyridyl porphyrins with amphiphilic N-substituents: fluorescent properties and photosensitized formation of singlet oxygen. J Appl Spec 80:813–823. doi:10.1007/s10812-014-9849-1
Steinhoff D, Weber H, Mohr U, Boehme K (1983) Evaluation of amitrole (aminotriazole) for potential carcinogenicity in orally dosed rats, mice, and golden hamsters. Toxicol Appl Pharm 69:161–169. doi:10.1016/0041-008X(83)90296-X
Sul'timova NB, Levin PP, Chaikovskaya ON, Sokolova IV (2008) Laser photolysis study of the triplet states of fulvic acids in aqueous solutions. High Energy Chem 42:464–468. doi:10.1134/S0018143908060088
Thurman EM (1985) Organic geochemistry of natural waters. M. Nijhoff and W. Junk Publishers, Dordrecht. doi:10.1007/978-94-009-5095-5
Tjälve H (1974) Fetal uptake and embryogenetic effects of aminotriazole in mice. Arch Toxicol 33:41–48. doi:10.1007/BF00297051
Watanabe N, Horikoshi S, Kawasaki A, Hidaka H (2005) Formation of refractory ring-expanded triazine intermediates during the photocatalyzed mineralization of the endocrine disruptor amitrole and related triazole derivatives at UV-irradiated TiO2/H2O interfaces. Environ. Sci. Technol. 39:2320–2326. doi:10.1021/es049791l
Weller C, Horn S, Herrmann H (2013) Effects of Fe(III)-concentration, speciation, excitation-wavelength and light intensity on the quantum yield of iron(III)-oxalato complex photolysis. J Photochem Photobiol A Chem 255:41–49. doi:10.1016/j.jphotochem.2013.01.014
Wilkinson F, Helman WP, Ross AB (1995) Rate constants for the decay and reactions of the lowest electronically excited singlet state of molecular oxygen in solution. An expanded and revised compilation. J Phys Chem Ref Data 24:663. doi:10.1063/1.555965
Zepp RG, Braun AM, Hoigné J, Leenheer JA (1987) Photoproduction of hydrated electrons from natural organic solutes in aquatic environments. Environ. Sci. Technol. 21:485–490. doi:10.1021/es00159a010
Acknowledgements
The work was financially supported by the Russian Foundation for Basic Research (grants no. 15-03-03376, 14-03-00692) and by Belorussian Republican Foundation for Fundamental Research (joint with SB RAS project BRFFR-SB RAS no. F15SО-036). HPLC analyses were performed with the financial support from the Grant Council of President of RF (MK-1515.2017.3).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Vítor Pais Vilar
Electronic supplementary material
ESM 1
(PDF 161 kb)
Rights and permissions
About this article
Cite this article
Pozdnyakov, I.P., Sherin, P.S., Salomatova, V.A. et al. Photooxidation of herbicide amitrole in the presence of fulvic acid. Environ Sci Pollut Res 25, 20320–20327 (2018). https://doi.org/10.1007/s11356-017-8580-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-8580-x