Abstract
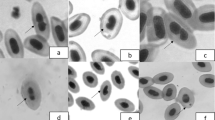
Although previous studies have already confirmed the toxicological potential of abamectin (ABA) in different experimental models (from invertebrates to vertebrates), almost nothing is known about the impacts the exposure to this pesticide can cause on birds. Thus, the aim of our study is to investigate the cytotoxic effects on the erythrocytes of female Japanese quails (Coturnix japonica) exposed to low abamectin concentrations. In order to do so, three experimental groups were proposed: “control,” composed of quails exposed to abamectin-free drinking water; “ABA 1% median lethal dose (LD50),” comprising birds exposed to water containing 15.5 mg a.i./L of abamectin (via commercial formulation Kraft® 36EC), and “ABA 10% LD50,” composed by birds exposed to water containing 155.0 mg a.i./L of abamectin. The micronucleus test and the tests applied to other nuclear abnormalities in the peripheral blood of birds were conducted 40 days after exposure. Our study revealed significant physical abnormalities in nuclear shapes (erythrocytes with asymmetric constriction nuclei, notched nuclei, indented and moved nucleus) of those birds exposed to higher abamectin levels. When all nuclear abnormalities were tallied, a significant dose-dependent trend was noted. Therefore, our study presents initial imprints on determination of abamectin-mediated cellular toxicity in avifauna which can be instrumental in checking polluted ecosystems.




Similar content being viewed by others
References
Abongwa M, Martin RJ, Robertson AP (2017) A brief review on the mode of action of antinematodal drugs. Acta Veterinaria 67:137–152
Alimba CG, Bakare AA (2016) In vivo micronucleus test in the assessment of cytogenotoxicity of landfill leachates in three animal models from various ecological habitats. Ecotoxicology 25:310–319
Baesse CQ, Tolentino VCM, Silva AM, Silva AA, Ferreira GA, Paniago LPM, Nepomuceno JC, Melo C (2015) Micronucleus as biomarker of genotoxicity in birds from Brazilian Cerrado. Ecotoxicol Environ Saf 115:223–228
Bhunya SP, Jena GB (1993) Studies on the genotoxicity of monocrotophos, an organophosphate insecticide, in the chick in vivo test system. Mutat Res 292:231–239
Braham RP, Blazer VS, Shaw CH, Mazik PM (2017) Micronuclei and other erythrocyte nuclear abnormalities in fishes from the Great Lakes Basin, USA. Environ Mol Mutagen 58(8):570–581
Campbell WC (1989) Ivermectin and Abamectin. Springer Verlag, New York
Carvalho FP (2017) Pesticide, environment, and food safety. Food Energy Security 6:48–60
El-Shafey AAM, Seliem MME, El-Mahrouky F, Gabr WM, Kandil RA (2011) Some physiological and biochemical effects of oshar extract and abamectin biocide on male albino rats. J Am Sci 7:254–261
Fisher MH, Mrozik H (1989) Chemistry. In: Campbell WC (ed) Ivermectin and Abamectin. Springer Verlag, New York
Fisher MH, Mrozik H (1992) The chemistry and pharmacology of avermectins. Annu Rev Pharmacol Toxicol 32:37–53
Gavrilescu M (2005) Fate of pesticide in the environment and its bioremediation. Engineering in Life Sci 5:497–526
Gómez-Meda BC, Zamora-Perez AL, Luna-Auirre J, González-Rodríguez A, Ramos-Ibarra L, Torres-Bugarín O, Batista-González CM, Zúñiga-González GM (2006) Nuclear abnormalities in erythrocytes of parrots (Aratinga canicularis) related to genotoxic damage. Avian Pathology 35:206–210
Hussain R, Mahmood F, Khan A, Javed MT, Rehan S, Mehdi T (2012) Cellular and biochemical effects induced by atrazine on blood of male Japanese quail (Coturnix japonica). Pestic Biochem Physiol 103:38–42
Hussain R, Khan A, Mahmood F, Rehan S, Ali F (2014) Clinico-hematological and tissue changes induced by butachlor in male Japanese quail (Coturnix japonica). Pestic Biochem Physiol 109:58–63
Hussain AG, Khan A, Abbas RZ, Asad M (2015) Clinico-hematological and mutagenic changes induced by arsenic and copper suplhate in adult poultry males. J Animal & Plant Sci 25:1555–1561
Hüter O (2011) Use of natural products in the crop protection industry. Phytochem Rev 10:185–194
Iarmarcovai G, Bonassi S, Botta A, Baan RA, Orsière T (2008) Genetic polymorphisms and micronucleus formation: a review of the literature. Mutat Res 658:215–253
Lajmanovich RC, Cabagna-Zenklusen MC, Attademo AM, Junges CM, Peltzer PM, Bassó A, Lorenzatti E (2014) Induction of micronuclei and nuclear abnormalities in tadpoles of the common toad (Rhinella arenarum) treated with the herbicides Liberty® and glufosinate-ammonium. Mutat Res Genet Toxicol Environ Mutagen 769:7–12
Lankas GR, Gordon LR (1989) Toxicology. In: Campbell WC (ed) Ivermectin and Abamectin. Springer Verlag, New York, pp 89–112
Lasota JA, Dybas RA (1991) Avermectins, a novel class of compounds: implications for use in arthropod pest control. Annu Rev Entomol 36:91–117
López González EC, Larriera A, Siroski PA, Poletta GL (2017) Micronuclei and other nuclear abnormalities on Caiman latirostris (Broad-snouted caiman) hatchlings after embryonic exposure to different pesticide formulations. Ecotoxicol Environ Saf 136:84–91. https://doi.org/10.1016/j.ecoenv.2016.10.035
MacGregor JT, Wehr CM, Henika PR, Shelby MD (1990) The in vivo erythrocyte micronucleus test: measurement at steady state increases assay efficiency and permits integration with toxicity studies. Fundam Appl Toxicol 14:513–522
Maioli MA, Medeiros HCD, Guelfi M, Trinca V, Pereira FTV, Mingatto FE (2013) The role of mitochondria and biotransformation in abamectin-induced cytotoxicity in isolated rat hepatocytes. Toxicol in Vitro 27:570–579
Ministério da Agricultura, Pecuária e Abastecimento (MAPA) – Brazil. (2017) Consulta de ingredient ativo. Avaiable in: http://agrofit.agricultura.gov.br/agrofit_cons/principal_agrofit_cons. Access in: 9 Nov., 2017
Montalvão MF, Souza JM, Guimarães ATB, Menezes IPP, Castro ALS, Rodrigues ASL, Malafaia G (2017) The genotoxicity and cytotoxicity of tannery effluent in bullfrog (Lithobates catesbeianus). Chemosphere 183:491–502
Nars HM, El-Demerdash FM, El-Nagar WA (2016) Neuro and renal toxicity induced by chlorpyrifos and abamectin in rats. Environ Sci Pollut Res 23:1852–1859
Novelli A, Vieira BH, Cordeiro D, Cappelini LT, Vieira EM, Espíndola EL (2012) Lethal effects of abamectin on the aquatic organisms Daphnia similis, Chironomus xanthus and Danio rerio. Chemosphere 86:36–40
Pollo FE, Grenat PR, Salinas ZA, Otero MA, Salas NE, Martino AL (2017) Evaluation in situ of genotoxicity and stress in South American common toad Rhinella arenarum in environments related to fluorite mine. Environ Sci Pollut Res 24:18179–18187
Schulte-Hostedde AI, Zinner B, Millar JS, Hickling GJ (2005) Restitution of mass-siza residuals: validating body condition indices. Ecology 86:155–163
Sharaf S, Khan A, Khan MZ, Aslam F, Saleemi MK, Mahmood F (2010) Clinico-hematological and micronuclear changes induced by cypermethrin in broiler chicks: their attenuation with vitamin E and selenium. Exp Toxicol Pathol 62:333–341
Skarphedinsdottir H, Hallgrimsson GT, Hansson T, Hägerroth PA, Liewenborg B, Tjärnlund U, Akerman G, Barsiene J, Balk L (2010) Genotoxicity in herring gulls (Larus argentatus) in Sweden and Iceland. Mutat Res 702:24–31
Souza JM, Montalvão MF, da Silva AR, Rodrigues ASL, Malafaia G (2017) A pioneering study on cytotoxicity in Australian parakeets (Melopsittacus undulates) exposed to tannery effluent. Chemosphere 175:521–533
Thanomsit C, Wattanakornsiri A, Nanthanawat P (2017) Adverse effects of abamectin on hematological profile and histological alterations of hybrid catfish (Clarias macrocephalus x C. gariepinus). Burapha Sci J 22:169–182
Torres-Bugarin O, Zavala-Cerna MG, Nava A, Flores- Garcia A, Ramos-Ibarra ML (2014) Potential uses, limitations, and basic procedures of micronuclei and nuclear abnormalities in buccal cells. Dis Markers 2014:956835
Vasconcelos AM, Daam MA, Santos LRA, Sanches ALM, Araújo CVM, Espíndola ELG (2016) Acute and chronic sensitivity, avoidance behavior and sensitive life stages of bullfrog tadpoles exposed to the biopesticide abamectin. Ecotoxicology 25:500–509
Wei L, Shao WW, Ding GH, Fan XL, ML Y, Lin ZH (2014) Acute and joint toxicity of three agrochemicals to Chinese tiger frog (Hoplobatrachus chinensis) tadpoles. Dongwuxue Yanjiu 35:272–279
Wolstenholme AJ (2012) Glutamate-gated chloride channels. J Biol Chem 287:40232–40238
Acknowledgments
The authors are grateful to the Brazilian National Council for Research (CNPq) (Brazilian research agency) (Proc. No. 467801/2014-2) and Instituto Federal Goiano for the financial support. Moreover, the authors are grateful to the CNPq for supporting scholarship to the student who developed this study.
Funding
This study was funded by the Brazilian National Council for Research (CNPq) (Brazilian research agency) (Proc. No. 467801/2014-2) and Instituto Federal Goiano – Campus Urutaí (GO, Brazil).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All the procedures were approved by The Ethics Committee on Animal Use of Goiano Federal Institute (Comissão de Ética no Uso de Animais do Instituto Federal Goiano), GO, Brazil (protocol No. 7257130516). Meticulous efforts were made to assure that the animals suffered the least possible and to reduce external sources of stress, pain, and discomfort. The current study did not exceed the number of animals necessary to produce trustworthy scientific data. This article does not contain any studies with human participants performed by any of the authors.
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
ESM 1
(DOCX 22 kb).
Rights and permissions
About this article
Cite this article
de Faria, D.B.G., Montalvão, M.F., de Souza, J.M. et al. Analysis of various effects of abamectin on erythrocyte morphology in Japanese quails (Coturnix japonica). Environ Sci Pollut Res 25, 2450–2456 (2018). https://doi.org/10.1007/s11356-017-0677-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0677-8