Abstract
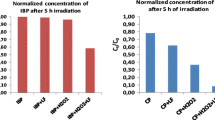
Many lipophilic pharmaceuticals may be sorbed in solid phases, leading to different photochemical behaviors. This study investigated the photochemistry of ciprofloxacin in a solid-phase system and compared it to that in a water-phase system. Kaolinite was used as the model solid matrix. The photolysis of ciprofloxacin in kaolinite fits pseudo-first-order kinetics for thicknesses less than 199 μm, and the rate constants k p decreased from 0.0154 to 0.0016 min−1 as the thickness of the layer increased. Unlike the aqueous phase, two-step degradation processes were observed for all kaolinite layer thicknesses (14–199 μm), and the pseudo-first-order constant at the surface of the kaolinite layer was smaller than that in the water phase. Comparatively, a similar photolysis rate constant of ciprofloxacin in a kaolinite suspension was also observed, and it was an order of magnitude smaller than that of the direct photodegradation (0.035 min−1) in water. The results indicate that ciprofloxacin is likely more stable when it is adsorbed on kaolinite and that the half-lives of ciprofloxacin in kaolinite and a kaolinite suspension are 2–25 times longer than that in deionized water (20 min) under simulated sunlight. Direct photolysis is proposed to be the main photodegradation mechanism for ciprofloxacin in kaolinite, and the cleavage of a piperazine ring is the main degradation pathway. However, the interaction between ciprofloxacin and kaolinite reduces the direct photolysis and leads to a higher light stability. In association with the reduction in photolysis, the yields of norfloxacin and defluorinated byproduct decreased significantly. Consequently, the interaction increases the persistence of ciprofloxacin and thus the ecological risk to the environment.





Similar content being viewed by others
References
Al-Ahmad A, Daschner FD, Kümmerer K (1999) Biodegradability of cefotiam, ciprofloxacin, meropenem, penicillin G, and sulfamethoxazole and inhibition of waste water bacteria. Arch Environ Con Tox 37:158–163. https://doi.org/10.1007/s002449900501
Babić S, Periša M, Škorić I (2013) Photolytic degradation of norfloxacin, enrofloxacin and ciprofloxacin in various aqueous media. Chemosphere 91:1635–1642. https://doi.org/10.1016/j.chemosphere.2012.12.072
Balmer ME, Goss K-U, Schwarzenbach RP (2000) Photolytic transformation of organic pollutants on soil surfaces an experimental approach. Environ Sci Technol 34:1240–1245
Brown KD, Kulis J, Thomson B, Chapman TH, Mawhinney DB (2006) Occurrence of antibiotics in hospital, residential, and dairy effluent, municipal wastewater, and the Rio Grande in New Mexico. Sci Total Environ 366:772–783. https://doi.org/10.1016/j.scitotenv.2005.10.007
Burhenne J, Ludwig M, Nikoloudis P, Spiteller M (1997a) Primary photoproducts and half-lives. Environ Sci Pollut Res 4:10–15. https://doi.org/10.1007/bf02986257
Burhenne J, Ludwig M, Spiteller M (1997b) Photolytic degradation of fluoroquinolone carboxylic acids in aqueous solution. Environ Sci Pollut Res 4:61–67. https://doi.org/10.1007/bf02986278
Ciani A, Goss K-U, Schwarzenbach RP (2005a) Photodegradation of organic compounds adsorbed in porous mineral layers: determination of quantum yields. Environ Sci Technol 39:6712–6720
Ciani A, Goss KU, Schwarzenbach RP (2005b) Light penetration in soil and particulate minerals. Eur J Soil Sci 56:561–574
da Silva MF, Tiago I, Veríssimo A, Boaventura RAR, Nunes OC, Manaia CM (2006) Antibiotic resistance of enterococci and related bacteria in an urban wastewater treatment plant. FEMS Microbiol Ecol 55:322–329. https://doi.org/10.1111/j.1574-6941.2005.00032.x
FDA (2015) National antimicrobial resistance monitoring scheme data tables. FDA http://www.fda.gov/downloads/AnimalVeterinary/SafetyHealth/AntimicrobialResistance/NationalAntimicrobialResistanceMonitoringSystem/UCM453387.pdf
Ferdig M, Kaleta A, Buchberger W (2005) Improved liquid chromatographic determination of nine currently used (fluoro)quinolones with fluorescence and mass spectrometric detection for environmental samples. J Sep Sci 28:1448–1456. https://doi.org/10.1002/jssc.200400065
Ferrari BT, Paxéus N, Giudice RL, Pollio A, Garric J (2003) Ecotoxicological impact of pharmaceuticals found in treated wastewaters: study of carbamazepine, clofibric acid, and diclofenac. Ecotox Environ Safe 55:359–370. https://doi.org/10.1016/S0147-6513(02)00082-9
Frost RL, Kristof J, Kloprogge JT, Horvath E (2000) Rehydration of potassium acetate-intercalated kaolinite at 298 K. Langmuir 16:5402–5408. https://doi.org/10.1021/la9915522
Ge L, Chen J, Wei X, Zhang S, Qiao X, Cai X, Xie Q (2010) Aquatic photochemistry of fluoroquinolone antibiotics: kinetics, pathways, and multivariate effects of main water constituents. Environ Sci Technol 44:2400–2405. https://doi.org/10.1021/es902852v
Girardi C, Greve J, Lamshöft M, Fetzer I, Miltner A, Schäffer A, Kästner M (2011) Biodegradation of ciprofloxacin in water and soil and its effects on the microbial communities. J Hazard Mater 198:22–30. https://doi.org/10.1016/j.jhazmat.2011.10.004
Golet EM, Alder AC, Giger W (2002) Environmental exposure and risk assessment of fluoroquinolone antibacterial agents in wastewater and river water of the Glatt Valley Watershed, Switzerland. Environ Sci Technol 36:3645–3651. https://doi.org/10.1021/es0256212
Hirsch R, Ternes T, Haberer K, Kratz K-L (1999) Occurrence of antibiotics in the aquatic environment. Sci Total Environ 225:109–118. https://doi.org/10.1016/S0048-9697(98)00337-4
Ji Y, Ferronato C, Salvador A, Yang X, Chovelon J-M (2014) Degradation of ciprofloxacin and sulfamethoxazole by ferrous-activated persulfate: implications for remediation of groundwater contaminated by antibiotics. Sci Total Environ 472:800–808
Jia H, Zhao J, Fan X, Dilimulati K, Wang C (2012) Photodegradation of phenanthrene on cation-modified clays under visible light. Appl Catal B-Environ 123:43–51. https://doi.org/10.1016/j.apcatb.2012.04.017
Katagi T (1993) Photodegradation of esfenvalerate in clay suspensions. J Agr Food Chem 41:2178–2183. https://doi.org/10.1021/jf00035a068
Kümmerer K, Henninger A (2003) Promoting resistance by the emission of antibiotics from hospitals and households into effluent. Clin Microbiol Infec 9:1203–1214. https://doi.org/10.1111/j.1469-0691.2003.00739.x
Li Z, Hong H, Liao L, Ackley CJ, Schulz LA, MacDonald RA, Mihelich AL, Emard SM (2011) A mechanistic study of ciprofloxacin removal by kaolinite. ColloidSurface B 88:339–344
Lin AY-C, Yu T-H, Lin C-F (2008) Pharmaceutical contamination in residential, industrial, and agricultural waste streams: risk to aqueous environments in Taiwan. Chemosphere 74:131–141. https://doi.org/10.1016/j.chemosphere.2008.08.027
Manaia CM, Novo A, Coelho B, Nunes OC (2010) Ciprofloxacin resistance in domestic wastewater treatment plants. Water Air Soil Poll 208:335–343. https://doi.org/10.1007/s11270-009-0171-0
McClellan K, Halden RU (2010) Pharmaceuticals and personal care products in archived U.S. biosolids from the 2001 EPA national sewage sludge survey. Water Res 44:658–668. https://doi.org/10.1016/j.watres.2009.12.032
Menager M, Sarakha M (2013) Simulated solar light phototransformation of organophosphorus azinphos methyl at the surface of clays and goethite. Environ Sci Technol 47:765–772. https://doi.org/10.1021/es301866f
Menager M, Siampiringue M, Sarakha M (2009) Photochemical behaviour of phenylbenzoquinone at the surface of the clays: kaolinite, bentonite and montmorillonite. J Photoch Photobio A 208:159–163. https://doi.org/10.1016/j.jphotochem.2009.09.010
Mountacer H, Atifi A, Sarakha M (2014) Degradation of the pesticide carbofuran on clay and soil surfaces upon sunlight exposure. Environ Sci Pollut Res Int 21:3443–3451
Moyo F, Tandlich R, Wilhelmi B, Balaz S (2014) Sorption of hydrophobic organic compounds on natural sorbents and organoclays from aqueous and non-aqueous solutions: a mini-review. Int J Environ Res Public Health 11:5020–5048
Picó Y, Andreu V (2007) Fluoroquinolones in soil—risks and challenges. Anal Bioanal Chem 387:1287–1299. https://doi.org/10.1007/s00216-006-0843-1
Sanderson H, Johnson DJ, Wilson CJ, Brain RA, Solomon KR (2003) Probabilistic hazard assessment of environmentally occurring pharmaceuticals toxicity to fish, daphnids and algae by ECOSAR screening. Toxicol Lett 144:383–395. https://doi.org/10.1016/S0378-4274(03)00257-1
Sayed M, Khan JA, Shah LA, Shah NS, Khan HM, Rehman F, Khan AR, Khan AM (2016) Degradation of quinolone antibiotic, norfloxacin, in aqueous solution using gamma-ray irradiation. Environ Sci Pollut Res 23:13155–13168. https://doi.org/10.1007/s11356-016-6475-x
Sturini M, Speltini A, Maraschi F, Profumo A, Pretali L, Fasani E, Albini A (2010) Photochemical degradation of marbofloxacin and enrofloxacin in natural waters. Environ Sci Technol 44:4564–4569. https://doi.org/10.1021/es100278n
Tolls J (2001) Sorption of veterinary pharmaceuticals in soils: a review. Environ Sci Technol 35:3397–3406. https://doi.org/10.1021/es0003021
Turiel E, Bordin G, Rodriguez AR (2005) Study of the evolution and degradation products of ciprofloxacin and oxolinic acid in river water samples by HPLC-UV/MS/MS-MS. J Environ Monitor 7:189–195. https://doi.org/10.1039/B413506G
Vazquez-Roig P, Segarra R, Blasco C, Andreu V, Picó Y (2010) Determination of pharmaceuticals in soils and sediments by pressurized liquid extraction and liquid chromatography tandem mass spectrometry. J Chromatogr A 1217:2471–2483. https://doi.org/10.1016/j.chroma.2009.11.033
Vieno NM, Härkki H, Tuhkanen T, Kronberg L (2007) Occurrence of pharmaceuticals in river water and their elimination in a pilot-scale drinking water treatment plant. Environ Sci Technol 41:5077–5084. https://doi.org/10.1021/es062720x
Wei X, Chen J, Xie Q, Zhang S, Ge L, Qiao X (2013) Distinct photolytic mechanisms and products for different dissociation species of ciprofloxacin. Environ Sci Technol 47:4284–4290. https://doi.org/10.1021/es400425b
Zhang X, Li R, Jia M, Wang S, Huang Y, Chen C (2015) Degradation of ciprofloxacin in aqueous bismuth oxybromide (BiOBr) suspensions under visible light irradiation: a direct hole oxidation pathway. Chem Eng J 274:290–297
Acknowledgments
We are grateful for the funding from the Ministry of Science and Technology (ROC) under grant 103-2221-E-002-240-MY5, which supported this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Suresh Pillai
Electronic supplementary material
ESM 1
(DOCX 1.23 mb)
Rights and permissions
About this article
Cite this article
Lin, YC., Hsiao, KW. & Lin, A.YC. Photolytic degradation of ciprofloxacin in solid and aqueous environments: kinetics, phototransformation pathways, and byproducts. Environ Sci Pollut Res 25, 2303–2312 (2018). https://doi.org/10.1007/s11356-017-0666-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0666-y