Abstract
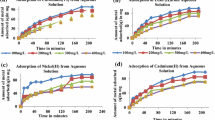
One of the most serious environmental issues of the present century is metal contamination of the aqueous environment due to the release of metal-containing effluents into the water bodies. Cadmium (Cd) is one of the toxic heavy metals which is not biodegradable thereby causing high risks to animals, plants, and humans. In the present study, potential and feasibility of compost derived from fruits and vegetables for Cd biosorption from aqueous solution were investigated. The batch biosorption experiments were performed to evaluate the effects of Cd concentrations (5, 15, 30, and 60 mg/L), compost biomass (0.5, 1.0, and 1.5 g/100 mL), pH (4, 6, and 8), contact time (1, 4, and 19 h), and temperature (28 and 35 °C) on Cd sorption and removal by compost. The biosorption of Cd was found to be highly dependent on initial Cd concentration, sorbent biomass, pH, contact time, and temperature of aqueous solution. It was observed that Cd sorption by compost was rapid up to 4 h, and then it became slow and stable as the contact time shifted towards equilibrium state (19 h). At equilibrium, the Cd sorption (q = 0.33–5.43 mg/g compost) and removal (45–99%) were observed at pH 6 and temperature 28 °C depending upon Cd concentrations and sorbent biomass in aqueous solution. The equilibrium experimental data were fitted well with Langmuir adsorption isotherm model (q max = 6.35–7.14 mg/g compost, R 2 = 0.77–0.98). FTIR spectrum of the compost indicated the presence of hydroxyl and carboxyl groups, which might be involved in the biosorption of Cd through ion exchange and complexation mechanisms. The optimal environmental conditions (pH 6, sorbent biomass 0.5 g/100 mL, and temperature 28 °C) induced more Cd sorption on compost at equilibrium. Results show compost as a cost-effective adsorbent material having high potential for heavy metal remediation from aqueous solution.






Similar content being viewed by others
References
Ahmad I, Akhtar MJ, Zahir ZA, Mitter B (2015) Organic amendments: effects on cereals growth and cadmium remediation. Int J Environ Sci Technol 12:2919–2928
Ali M, Bhatia A, Kazmi AA, Ahmed N (2012) Characterization of high rate composting of vegetable market waste using Fourier transform-infrared (FT-IR) and thermal studies in three different seasons. Biodegradation 23:231–242
Anagnostopoulos VA, Koutsoukos PG, Symeopoulos BD (2015) Removal of U (VI) from aquatic systems, using winery by-products as biosorbents: equilibrium, kinetic, and speciation studies. Water Air Soil Pollut 226:1–14
Anagnostopoulos V, Symeopoulos B, Bourikas K, Bekatorou A (2016) Biosorption of U (VI) from aqueous systems by malt spent rootlets: kinetic, equilibrium and speciation studies. Int J Environ Sci Technol 13:285–296
Babarinde A, Ogundipe K, Sangosanya KT, Akintola BD, Hassan AOE (2016) Comparative study on the biosorption of Pb(II), Cd(II) and Zn(II) using lemon grass (Cymbopogon citratus): kinetics, isotherms and thermodynamics. Chem Int 2:89–102
Babel S, Kurniawan TA (2003) Low-cost adsorbents for heavy metals uptake from contaminated water: a review. J Hazard Mater 28:219–243
Barakat M (2011) New trends in removing heavy metals from industrial wastewater. Arab J Chem 4:361–377
Bilal M, Shah JA, Ashfaq T, Gardazi SMH, Tahir AA, Pervez A, Haroon H, Mahmood Q (2013) Waste biomass adsorbents for copper removal from industrial wastewater—a review. J Hazard Mater 263:322–333
Clemente R, Bernal MP (2006) Fractionation of heavy metals and distribution of organic carbon in two contaminated soils amended with humic acids. Chemosphere 64:1264–1273
Crini G (2006) Non-conventional low-cost adsorbents for dye removal: a review. Bioresour Technol 97:1061–1085
Feng N, Guo X, Liang S, Zhu Y, Liu J (2011) Biosorption of heavy metals from aqueous solutions by chemically modified orange peel. J Hazard Mater 185:49–54
Ghorbani M, Eisazadeh H, Ghoreyshi A (2012) Removal of zinc ions from aqueous solution using polyaniline nanocomposite coated on rice husk. Iran J Energy Environ 3:83–88
Hai FI, Yamamoto K, Fukushi K (2007) Hybrid treatment systems for dye wastewater. Crit Rev Environ Sci Technol 37:315–377
Hameed B, Din AM, Ahmad A (2007) Adsorption of methylene blue onto bamboo-based activated carbon: kinetics and equilibrium studies. J Hazard Mater 141:819–825
Hanif MA, Nadeem R, Bhatti HN, Ahmad NR, Ansari TM (2007) Ni (II) biosorption by Cassia fistula (Golden Shower) biomass. J Hazard Mater 139:345–355
Henryk K, Jarosław C, Witold Ż (2016) Peat and coconut fiber as biofilters for chromium adsorption from contaminated wastewaters. Environ Sci Pollut Res 23:527–534
Hussein BI (2010) Removal of copper ions from waste water by adsorption with modified and unmodified sunflower stalks. J Eng 16:5411–5421
Keijzer T, Pijls C, Marnette E, Sumann M, Folkering F, Zutphen M (2006) In situ soil and groundwater remediation: theory and practice. Tauw bv, AC Deventer. Netheralnds.
Lin S-H, Juang R-S (2009) Adsorption of phenol and its derivatives from water using synthetic resins and low-cost natural adsorbents: a review. J Environ Manag 90:1336–1349
Lupea M, Bulgariu L, Macoveanu M (2012) Biosorption of Cd(II) from aqueous solution on marine green algae biomass. Environ Eng Manag J 11:607–615
Martinho J, Campos B, Brás I, Silva E (2015) The role of compost properties in sorption of heavy metals. Environ Protec Eng 41:57–65
Matouq M, Jildeh N, Qtaishat M, Hindiyeh M, Al Syouf MQ (2015) The adsorption kinetics and modeling for heavy metals removal from wastewater by Moringa pods. J Environ Chem Eng 3:775–784
Moore JW, Ramamoorthy S (2012) Heavy metals in natural waters: applied monitoring and impact assessment. Springer Science & Business Media. pp 268.
Naiya TK, Chowdhury P, Bhattacharya AK, Das SK (2009) Saw dust and neem bark as low-cost natural biosorbent for adsorptive removal of Zn(II) and Cd(II) ions from aqueous solutions. Chem Eng J 148:68–79
Nandi B, Goswami A, Purkait M (2009) Removal of cationic dyes from aqueous solutions by kaolin: kinetic and equilibrium studies. Appl Clay Sci 42(3–4):583–590
Ofomaja AE (2008) Kinetic study and sorption mechanism of methylene blue and methyl violet onto mansonia (Mansonia altissima) wood sawdust. Chem Eng J 143:85–95
Panday K, Prasad G, Singh V (1985) Copper (II) removal from aqueous solutions by fly ash. Water Res 19:869–873
Peralta-Videa JR, Lopez ML, Narayan M, Saupe G, Gardea-Torresdey J (2009) The biochemistry of environmental heavy metal uptake by plants: implications for the food chain. Int J Biochem Cell Biol 41:1665–1677
Poulopoulos S, Inglezakis, VJ (2006) Adsorption, ion exchange and catalysis: design of operations and environmental applications. Elsevier, Amsterdam.
Raoof A, Hassanizadeh SM, Leijnse A (2010) Upscaling transport of adsorbing solutes in porous media: pore-network modeling. Vadose Zone J 9:624–636
Reddad Z, Gerente C, Andres Y, Le Cloirec P (2002) Adsorption of several metal ions onto a low-cost biosorbent: kinetic and equilibrium studies. Environ Sci Technol 36:2067–2073
Reddy DHK, Seshaiah K, Reddy A, Lee S (2012) Optimization of Cd (II), Cu (II) and Ni (II) biosorption by chemically modified Moringa oleifera leaves powder. Carbohydr Polym 88:1077–1086
Sai W, Kaewsarn P, Saikaew W (2009) Pomelo peel: agricultural waste for biosorption of cadmium ions from aqueous solutions. World Acad Sci Eng Technol 56:287–291
Santhi T, Manonmani S, Smitha T (2010) Kinetics and isotherm studies on cationic dyes adsorption onto annona squmosa seed activated carbon. Int J Eng Sci Technol 2:287–295
Seelsaen N, McLaughlan R, Moore S, Stuetz R (2007) Influence of compost characteristics on heavy metal sorption from synthetic stormwater. Water Sci Technol 55:219–226
Shawabkeh RA, Rockstraw DA, Bhada RK (2002) Copper and strontium adsorption by a novel carbon material manufactured from pecan shells. Carbon 40:781–786
Singh R, Gautam N, Mishra A, Gupta R (2011) Heavy metals and living systems: an overview. Indian J Pharmacol 43:246–253
Sud D, Mahajan G, Kaur M (2008) Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions—a review. Bioresour Technol 99:6017–6027
Sumathi T, Alagumuthu G (2014) Adsorption studies for arsenic removal using activated Moringa oleifera. Int J Chem Eng. doi:10.1155/2014/430417
Tofan L, Paduraru C, Volf I (2011) Maize bran as a low-cost resource for Cu(II) ions removal. Cellulose. Chem Technol 45:275–280
Vijayaraghavan K, Yun Y-S (2008) Bacterial biosorbents and biosorption. Biotechnol Adv 26:266–291
Volesky B (2007) Biosorption and me. Water Res 41:4017–4029
Wahab MA, Jellali S, Jedidi N (2010) Ammonium biosorption onto sawdust: FTIR analysis, kinetics and adsorption isotherms modeling. Bioresour Technol 101:5070–5075
Wang J, Chen C (2006) Biosorption of heavy metals by Saccharomyces cerevisiae: a review. Biotechnol Adv 24:427–451
Wang J, Chen C (2009) Biosorbents for heavy metals removal and their future. Biotechnol Adv 27:195–226
Wong K, Lee CK, Low KS, Haron M (2003) Removal of Cu and Pb by tartaric acid modified rice husk from aqueous solutions. Chemosphere 50:23–28
Wuana RA, Okieimen FE (2011) Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol. doi:10.5402/2011/402647
Yao S, Lai H, Shi Z (2012) Biosorption of methyl blue onto tartaric acid modified wheat bran from aqueous solution. Iran J Environ Health Sci Eng 9:1–6
Yasin Y, Hussein MZ, Ahmad H (2007) Adsorption of methylene blue onto treated activated carbon. The Malays J Analy Sci 11:400–406
Zhou Y-F, Haynes R-J (2010) Sorption of heavy metals by inorganic and organic components of solid wastes: significance to use of wastes as low-cost adsorbents and immobilizing agents. Crit Rev Environ Sci Technol 40:909–977
Acknowledgements
The authors are grateful to Professor Dr. Zahir Ahmad Zahir for providing instruments and space for conducting this study, which was partially funded by the Higher Education Commission of Pakistan under “Indigenous 5000 Fellowship Scheme given to first author.”
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Marcus Schulz
Rights and permissions
About this article
Cite this article
Ahmad, I., Akhtar, M.J., Jadoon, I.B.K. et al. Equilibrium modeling of cadmium biosorption from aqueous solution by compost. Environ Sci Pollut Res 24, 5277–5284 (2017). https://doi.org/10.1007/s11356-016-8280-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-8280-y