Abstract
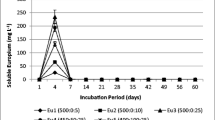
Due to their characteristics, colloidal particles are able to control the dispersion of many organic and inorganic pollutants in soils and streams. Colloidal precipitates generated by acid mine drainage (AMD) process are usually amorphous or nanocrystalline materials, and their stability plays a crucial role in controlling the fate of metals released by sulphide oxydation. This paper describes a study of elements release (Fe, Al, Mn, Cd, Co, Cr, Cu, Ni, S, Zn) due to desorption or destabilization of three different colloidal precipitates, two ochreous and a greenish-blue precipitate, sampled at the Libiola mine site (northwest Italy). The samples were heated at high temperature in order to verify this treatment as inertization process. At room temperature, the most easily extracted element was S (with released percentages from 8.39 to 29.17 %), but considerable amounts of Cu, Zn and Mn (up to 16.6, 610.6 and 595.6 mg/kg, respectively) were also observed in the leachates for greenish-blue precipitates. The highest release of elements (S > Cu, Zn, Mn, Cd > Co, Ni > Al, Fe, Cr), with minor differences depending on the mineralogical composition of the samples, was observed for heat-treated samples obtained through moderate heating and mainly formed by anhydrous phases. Samples treated at high temperature had the lowest release, with only Cu showing a significant concentration in the leachate of greenish-blue precipitates. The results showed that dissolution/desorption is limited from ochreous natural colloidal precipitates occurring at the Libiola mine site but also that high amounts of some metals can be remobilized from greenish-blue precipitates. The destabilization of all percipitates through dehydratation–dehydroxylation can further remobilize important amounts of ecotoxic elements. Heat treatment at high temperature could be a definitive, although expensive, way to fix heavy metals in the solid fraction, preventing their dispersion in the surrounding environment.








Similar content being viewed by others
References
Accornero M, Marini L, Ottonello G, Zuccolini MV (2005) The fate of major constituents and chromium and other trace elements when acid waters from the derelict Libiola mine (Italy) are mixed with stream waters. Appl Geochem 20:1368–1390
Acero P, Ayora C, Torrentó C, Nieto JM (2006) The behavior of trace elements during schwertmannite precipitation and subsequent transformation into goethite and jarosite. Geochim Cosmochim Acta 70:4130–4139
Ardau C, Frau F, Dore E, Lattanzi P (2012) Molybdate sorption by Zn–Al sulphate layered double hydroxides. Appl Clay Sci 65–66:128–133
Badocco D, Lavagnini I, Mondin A, Tapparo A, Pastore P (2015) Limit of detection in the presence of instrumental and non-instrumental errors: study of the possible sources of error and application to the analysis of 41 elements at trace levels by inductively coupled plasma-mass spectrometry technique. Spectrochim Acta 107:178–184
Balistrieri LS, Seal RR II, Piatak NM, Paul (2007) Assessing the concentration, speciation, and toxicity of dissolved metals during mixing of acid-mine drainage and ambient river water downstream of the Elizabeth Copper Mine, Vermont, USA. Appl Geochem 22:930–952
Bera P, Rajamathi M, Hedge MS, Kamath PV (2000) Thermal behaviour of hydroxides, hydroxysalts and hydrotalcites. Bull Mater Sci 23:141–145
Bigham JM (1994) Mineralogy of ochre deposits formed by sulphide oxidation. In: Jambor JL , Blowes DW (eds) Environmental geochemistry of sulphide mine-waters. Mineralogical Association of Canada, Short Course Handbook 22 on Environmental Geochemistry of Sulfide Mine Wastes, Waterloo, Ontario, pp 103–132
Bigham J, Carlson L, Murad E (1994) Schwertmannite, a new iron oxyhydroxysulphate from Pyhäsalmi, Finland, and other localities. Mineral Mag 58:641–648
Blowes DW, Ptacek CJ, Jambor JL, Weisener CG, Paktunc D, Gould WD, Johnson DB (2014) The geochemistry of acid mine drainage. In: Turekian KK, Holland HD (eds), Treatise of Geochemistry 2nd Edition, volume 11, Elsevier, Amsterdam, pp 131–190
Burgos WD, Borch T, Troye LD, Luan F, Larson LN, Brown JF, Lambson J, Shimizu M (2012) Schwertmannite and Fe oxides formed by biological low-pH Fe(II) oxidation versus abiotic neutralization: impact on trace metal sequestration. Geochim Cosmochim Acta 76:29–44
Carbone C, Di Benedetto F, Marescotti P, Martinelli A, Sangregorio C, Cipriani C, Lucchetti G, Romanelli M (2005) Genetic evolution of nanocrystalline Fe oxide and oxyhydroxide assemblages from the Libiola Mine (eastern Liguria, Italy): structural and microstructural investigations. Eur J Mineral 17:785–795
Carbone C, Marescotti P, Lucchetti G, Cauzid J, Chalmin E (2011) Application of synchrotron radiation-based techniques (μ-XRD, μ-XRF, and μ-XANES) to study Fe-rich hardpans within waste-rock dump. NJMA Spec Issue 188(1):21–30
Carbone C, Dinelli E, Marescotti P, Gasparotto G, Lucchetti G (2013) The role of AMD secondary minerals in controlling environmental pollution: indications from bulk leaching tests. J Geochem Explor 132:188–200
Cashion JD, Murad E (2012) Mössbauer spectra of the acid mine drainage mineral Schwertmannite from the Sokolov Basin, Czech Republic. In: Proceedings of the 36th annual Condensed Matter and Materials Meeting, 31 Jan. -3 Feb. 2012. Wagga Wagga, NSW, Australia
Cavani F, Trifiro F, Vaccari A (1991) Hydrotalcite-type anionic clays: preparation, properties and applications. Catal Today 11:173–301
Chen YH (2013) Thermal properties of nanocrystalline goethite, magnetite, and maghemite. J Alloys Compd 553:194–198
Cidu R, Frau F (2009) Distribution of trace elements in filtered and non filtered aqueous fractions: insights from rivers and streams of Sardinia (Italy). Appl Geochem 24:611–623
Cornell RM, Schwertmann U (2003) The iron oxides: structure, properties, reactions, occurrences and uses, 2nd edn. Wiley-VCH, Weinheim
Dinelli E, Tateo F (2002) Different types of fine-grained sediments associated with acid mine drainage in the Libiola Fe–Cu mine area (Ligurian Apennines, Italy). Appl Geochem 17:1081–1092
Dinelli E, Morandi N, Tateo F (1998) Fine grained weathering products in waste disposal from two sulphide mines in the northern Appennines, Italy. Clay Miner 33:423–433
Dinelli E, Lucchini F, Fabbri M, Cortecci G (2001) Metal distribution and environmental problems related to sulfide oxidation in the Libiola copper mine area (Ligurian Apennines, Italy). J Geochem Explor 74:141–152
Dold B, Fontboté L (2001) Element cycling and secondary mineralogy in porphyry copper tailings as a function of climate, primary mineralogy, and mineral processing. J Geochem Explor 74:3–15
Dormann JL, Fiorani D, Tronc E (1997) Magnetic relaxation in fine-particle system. Adv Chem Phys 98:283–494
Drouet C, Navrotsky A (2003) Synthesis, characterization, and thermochemistry of K-Na-H3O jarosites. Geochim Cosmochim Acta 67:2063–2076
Frau F, Ardau C (2004) Mineralogical controls on arsenic mobility in the Baccu Locci stream catchment (Sardinia, Italy) affected by past mining. Mineral Mag 68:15–30
Frau F, Biddau R, Fanfani L (2008) Effect of major anions on arsenate desorption from ferrihydrite-bearing natural samples. Appl Geochem 23:1451–1466
Frau F, Addari D, Atzei D, Biddau R, Cidu R, Rossi A (2010) Influence of major anions on As(V) adsorption by synthetic 2-line ferrihydrite. Kinetic investigation and XPS study of the competitive effect of bicarbonate. Water Air Soil Pollut 205:25–41
Frost RL, Wain D, Martens WN, Locke AC, Martinez-Frias J, Rull F (2007) Thermal decomposition and X-ray diffraction of sulphate efflorescent minerals from El Jaroso Ravine, Sierra Almagrera, Spain. Thermochim Acta 460:9–14
Furrer G, Phillips BL, Ulrich K-U, Pöthig R, Casey WH (2002) The origin of aluminum flocs in polluted streams. Science 297:2245–2247
Hageman PL, Briggs PH (2000) A simple field leach for rapid screening and qualitative characterization of mine-waste material on abandoned mine lands. In: Proceedings from the Fifth International Conference on Acid Rock Drainage, Denver, Colorado, May 21–24, 2000, vol. II. Society for Mining, Metallurgy and Exploration, Inc., pp 1463–1475
Hageman PL, Seal RR, Diehl SF, Piatak NM, Lowers HA (2015) Evaluation of selected static methods used to estimate element mobility, acid-generating and acid-neutralizing potentials associated with geologically diverse mining wastes. Appl Geochem 57:125–139
Henmi T, Tange K, Minagawa T, Yoshinaga N (1981) Effect of SiO2/A12O3 ratio on the thermal reactions of allophane. II. Infrared and x-ray powder diffraction data. Clay Clay Miner 29:124–128
ISPRA (2011) Carta Geologica d’Italia 1:25.000, Foglio 232 Sestri Levante. http://www.isprambiente.gov.it/Media/carg/232_SESTRI_LEVANTE/Foglio.html
Jambor JL, Dutrizac JE (1998) Occurrence and constitution of natural and synthetic ferrihydrite, a widespread iron oxyhydroxide. Chem Rev 98:2549–2585
Jönsson J, Söjberg S, Lövgren L (2006) Adsorption of Cu(II) to schwertmannite and goethite in presence of dissolved organic matter. Water Res 40:969–974
Kaplan DJ, Bertsch PM, Adriano DD, Orlandini KA (1994) Actinide association with groundwater colloids in a coastal plain aquifer. Radiochim Acta 66/67:181–187
Kim Y (2015) Mineral phases and mobility of trace metals in white aluminum precipitates found in acid mine drainage. Chemosphere 119:803–811
Kim JJ, Kim SJ (2003) Environmental, mineralogical, and genetic characterization of ochreous and white precipitates from Acid Mine Drainages in Taebaeg, Korea. Environ Sci Technol 37:2120–2126
Kosmulski M (2009) Surface charging and points of zero charge. Surfactant science series, vol 145. CRC Press, Boca Raton
Landers M, Gilkes RJ (2007) Dehydroxylation and dissolution of nickeliferous goethite in New Caledonian lateritic Ni ore. Appl Clay Sci 35:162–172
Leclerc A (1980) Room temperature Mössbauer analysis of jarosite-type compounds. Phys Chem Miner 6:327–334
Leivo J, Lindén M, Ritola M, Vippola M, Levänen E, Mäntylä TA (2009) Influence of thermal treatment conditions on the formation of phase-pure mullite derived from a nanoparticulate aluminosilicate precursor. Mater Chem Phys 115:56–64
Liu H, Chen T, Zou X, Qing C, Frost RL (2013) Thermal treatment of natural goethite: thermal transformation and physical properties. Thermochim Acta 568:115–121
Liu H, Chen T, Frost RL (2014) An overview of the role of goethite surfaces in the environment. Chemosphere 103:1–11
Lowry GV, Shaw S, Kim CS, Rytuba JJ, Brown GE Jr (2004) Macroscopic and microscopic observations of particle-facilitated mercury transport from New Idria and Sulphur Bank mercury mine tailings. Environ Sci Technol 38:5101–5111
Marescotti P, Carbone C, Comodi P, Frondini F, Lucchetti G (2012) Mineralogical and chemical evolution of ochreous precipitates from the Libiola Fe–Cu-sulfide mine (Eastern Liguria, Italy). Appl Geochem 27:577–589
Miyata S, Okada A (1977) Synthesis of hydrotalcite-like compounds and their physico-chemical properties – the systems Mg2+-AI3+-SO4 2− and Mg2+-A13+-CrO4 2−. Clay Clay Miner 25:14–18
Munk L, Faure G, Pride DE, Bigham JM (2002) Sorption of trace metals to an aluminum precipitate in a stream receiving acid rock-drainage; Snake River, Summit County, Colorado. Appl Geochem 17:421–430
Murad E, Johnston JH (1987) Iron oxides and oxyhydroxides. In: Long GJ (ed) Mössbauer spectroscopy applied to inorganic chemistry, vol 2. Plenum Press, New York, p 523
Nickel EH (1976) New data on woodwardite. Mineral Mag 43:644–647
Nodari L, Centomo P, Salviulo G, Russo U (2008) Synthesis and characterization of iron(III) oxides as supports for Au(0)-based catalysts. Mater Chem Phys 108:237–246
Parker SR, Gammons CH, Jones CA, Nimick DA (2007) Role of hydrous iron oxide formation in attenuation and diel cycling of dissolved trace metals in a stream affected by acid rock drainage. Water Air Soil Pollut 181:247–263
Ptacek CJ, Blowes DW (2003) Geochemistry of concentrated waters at mine-wastes sites. In: Environmental Aspect of Mine Wastes. Mineralogical Association of Canada, Short Course Series 31, Vancouver, British Columbia (Canada), pp 239–260
Regenspurg S, Brand A, Peiffer S (2004) Formation and stability of schwertmannite in acidic mining lakes. Geochim Cosmochim Acta 68:1185–1197
Schemel LE, Kimball BA, Bencala KE (2000) Colloid formation and metal transport through two mixing zones affected by acid mine drainage near Silverton, Colorado. Appl Geochem 15:1003–1018
Schwertmann U, Friedl J, Stanjek H (1999) From Fe(III) ions to ferrihydrite and then to hematite. J Colloid Interface Sci 209:215–223
Serrano S, Gomez-Gonzalez MA, O’Day PA, Laborda F, Bolea E, Garrido F (2015) Arsenic speciation in the dispersible colloidal fraction of soils from amine-impacted creek. J Hazard Mater 286:30–40
Sidhu PS (1988) Transformation of trace elements-substituted maghemite to hematite. Clay Clay Miner 36:31–38
Sidhu PS, Gilkes RJ, Posner AM (1980) The behaviour of Co, Ni, Zn, Cu, Mn, and Cr in magnetite during alteration to maghemite and hematite. Soil Sci Soc Am J 44:135–138
Stipp SLS, Hansen M, Kristensen R, Hochella MF Jr, Bennedsen L, Dideriksen K, Balic-Zunic T, Léonard D, Mathieu H-J (2002) Behaviour of Fe-oxides relevant to contaminant uptake in the environment. Chem Geol 190:321–337
Stumm W, Morgan JJ (1996) Aquatic chemistry, 3rd edn. John Wiley and Sons, Inc, New York
Tumiati S, Godard G, Masciocchi N, Martin S, Monticelli D (2008) Environmental factors controlling the precipitation of Cu-bearing hydrotalcite-like compounds from mine waters. The case of the “Eve verda” spring (Aosta Valley, Italy). Eur J Mineral 20:73–94
Wada K (1989) Allophane and imogolite. In: Minerals in soil environment, 2nd edn. Soil Sci Soc Am 1051–1087
Walton-Day K (2003) Passive and active treatment of mine drainage. In: Jambor JL, Blowes DW, Ritchie AIM (eds) Environmental aspects of mine wastes. Mineralogical Association of Canada Short Course Handbook 31, Vancouver, British Columbia, pp 335–359
Witzke T (1999) Hydrowoodwardite, a new mineral of the hydrotalcite group from Königswalde near Annaberg, Saxony/Germany and other localities. N Jb Mineral Mh 2:75–86
Yuan G, Percival HJ, Theng BKG, Parfitt RL (2002) Sorption of copper and cadmium by allophane-humic complexes. In: Violante A, Huang PM, Bollag JM, Gianfreda L (eds) Soil mineral-organic matter-microorganism interactions and ecosystem health. Dev Soil Sci 28A:37–47
Zaccarini F, Garuti G (2008) Mineralogy and chemical composition of VMS deposits of northern Apennine ophiolites, Italy: evidence for the influence of country rock type on ore composition. Mineral Petrol 94:61–83
Acknowledgments
This study was funded by MIUR – (Italian) Ministero dell’Istruzione, dell’Università e della Ricerca (PRIN-COFIN 2010-2011): “Minerals-Biosphere Interaction: Environmental and Health Consequences.” Dr. Nodari is grateful to Department of Chemical Science, University of Padua, for allowing him the use of Mössbauer apparatus.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Consani, S., Carbone, C., Salviulo, G. et al. Effect of temperature on the release and remobilization of ecotoxic elements in AMD colloidal precipitates: the example of the Libiola copper mine, Liguria, (Italy). Environ Sci Pollut Res 23, 12900–12914 (2016). https://doi.org/10.1007/s11356-016-6406-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6406-x