Abstract
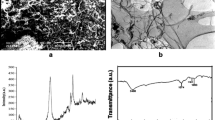
Silica combined with 2 % multiwall carbon nanotubes (SiO2-CNT) was synthesized and characterized. Its sorption efficacy was investigated for the Hg(II) removal from an aqueous solution. The effect of pH on the percentage removal by the prepared material was examined in the range from 3 to 7. The adsorption kinetics were well fitted by using a pseudo-second-order model at various initial Hg(II) concentrations with R 2 of >0.99. The experimental data were plotted using the interparticle diffusion model, which indicated that the interparticle diffusion is not the only rate-limiting step. The data is well described by the Freundlich isotherm equation. The activation energy (Ea) for adsorption was 12.7 kJ mol−1, indicating the process is to be physisorption. Consistent with an endothermic process, an increase in the temperature resulted in increasing mercury removal with a ∆Ho of 13.3 kJ/mol and a ∆So 67.5 J/mol K. The experimental results demonstrate that the combining of silica and nanotubes is a promising alternative material, which can be used to remove the mercury from wastewaters.













Similar content being viewed by others
References
Anderson DR (1974) In: Lee Smith A (ed) Analysis of Silicones. Wiley-Intersciences, New York, Chapter 10
Anirudhan TS, Divya L, Ramachandran M (2008) Mercury(II) removal from aqueous solutions and wastewaters using a novel cation exchanger derived from coconut coir pith and its recovery. J Hazard Mater 157:620–627
Cui H, Qian Y, Li Q, Zhang Q, Zhai J (2012) Adsorption of aqueous Hg(II) by a polyaniline/attapulgite composite,”. Chem Eng J 211–212:216–223
El-Gamel EA, Wortmann L, Arroubb K, Mathur S (2011) SiO2@Fe2O3 core–shell nanoparticles for covalent immobilization and release of sparfloxacin drugw. Chem Commun 47:10076–10078
Freundlich H (1906) Uber die adsorption in losungen (adsorption in solution). Z Phys Chem 57:384–470
Fu X, Feng X, Sommar J, Wang S (2012) A review of studies on atmospheric mercury in China. Sci Total Environ 421–422:73–81
Iijima S (1991) Helical microtubes of graphitic carbon. Nature 354:56–58
Insin N, Tracy JB, Lee H, Zimmer JP, Westervelt RM, Bawendi MG (2008) Incorporation of iron oxide nanoparticles and quantum dots into silica microspheres. ACS Nano 2:197–202
Kadirvelu K, Kavipriya M, Karthika C, Vennilamani N, Pattabhi S (2004) Mercury (II) adsorption by activated carbon made from sago waste. Carbon 42:745–752
Kadirvelu K, Goel J, Rajagopal C (2008) Sorption of lead, mercury and cadmium ions in multi-component system using carbon aerogel as adsorbent. J Hazard Mater 153:502–507
Kim MH, Na HK, Kim YK, Ryoo SR, Cho HS, Lee KE, Jeon H, Ryoo R, Min DH (2011) Facile synthesis of monodispersed mesoporous silica nanoparticles with ultralarge pores and their application in gene delivery. ACS Nano 5:3568–3576
Knocke WR, Hemphill LH (1981) Mercury(II) sorption by waste rubber. Water Res 15:275–282
Labidi NS (2008) Removal of mercury from aqueous solutions by waste brick. Int J Environ Res 2:275–278
Lagergren S (1898) About the theory of so-called adsorption of solution substances, kunglia srenska vertens Ka psakademiens Handlingar. 24: 1–39
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1362–1403
Mehdinia A, Akbari M, Baradaran T, Azad M (2015) High-efficient mercury removal from environmental water samples using di-thio grafted on magnetic mesoporous silica nanoparticles. Environ Sci Pollut Res 22:2155–2165
Saifuddin N, Raziah AZ (2007) Removal of heavy metals fromindustrialeffluent using Saccharomyces cerevisiae (Baker’s yeast) immobilized in chitosan/lignosulphonate matrix. J Appl Sci Res 3:2091–2099
Saikia BJ, Parthasarathy G (2010) Fourier transform infrared spectroscopic characterization of kaolinite from Assam and Meghalaya, Northeastern India. J Mod Phys 1:206–210
Saleh TA (2011) The influence of treatment temperature on the acidity of MWCNT oxidized by HNO3 or a mixture of HNO3/H2SO4. Appl Surf Sci 257(17):7746–7751
Saleh TA, Gupta VK (2011) Functionalization of tungsten oxide into MWCNT and its application for sunlight-induced degradation of rhodamine B. J Colloid Interface Sci 362(2):337–344
Saleh TA, Gupta VK (2014) Processing methods, characteristics and adsorption behavior of tire derived carbons: a review. Adv Colloid Interf Sci 211:93–101
Sari A, Tuzen M (2009) Removal of mercury(II) from aqueous solution using moss (Drepanocladus revolvens) biomass: Equilibrium, thermodynamic and kinetic studies. J Hazard Mater 171:500–507
Shadbad MJ, Mohebbi A, Soltani A (2011) Mercury(II) removal from aqueous solutions by adsorption on multi-walled carbon nanotubes. Korean J Chem Eng 28:1029–1034
Shawky HA, El-Aassar A, Abo-Zeid DE (2012) Chitosan/carbon nanotube composite beads: Preparation, characterization, and cost evaluation for mercury removal from wastewater of some industrial cities in Egypt. J Appl Polym Sci 125:E93–E101
Treybal RE (1968) Mass Transfer Operations, 2nd edn. McGraw Hill, New York
Unuabonah EI, Adebowale KO, Olu-Owolabi BO (2007) Kinetic and thermodynamic studies of the adsorption of lead (II) ions onto phosphate-modified kaolinite clay. J Hazard Mater 144:386–395
Vasudevan S, Lakshmi J, Sozhan G (2012) Optimization of electrocoagulation process for the simultaneous removal of mercury, lead, and nickel from contaminated water. Environ Sci Pollut Res 19:2734–2744
Wang J, Feng X, Anderson CWN, Xing Y, Shang L (2012) Remediation of mercury contaminated sites—a review. J Hazard Mater 221–222:1–18
Wang Q, Qin W, Chai L, Li Q (2014) Understanding the formation of colloidal mercury in acidic wastewater with high concentration of chloride ions by electrocapillary curves. Environ Sci Pollut Res 21:3866–3872
Webber TN, Chakravarti RK (1974) Pore and solid diffusion models for fixed bed adsorbers. J Am Inst Chem Eng 20:228–238
Yardim MF, Budinova T, Ekinci E, Petrov N, Razvigorova M, Minkova V (2003) Removal of mercury (II) from aqueous solution by activated carbon obtained from furfural. Chemosphere 52:835–841
Yin ZH, Liu X, Su ZX (2010) Novel fabrication of silica nanotubes using multi-walled carbon nanotubes as template. Bull Mater Sci 33:351–355
Yu Y, Addai-Mensah J, Losic D (2012) Functionalized diatom silica microparticles for removal of mercury ions. Sci Technol Adv Mater 13:015008 (11pp)
Zhang M, Wu Y, Feng X, He X, Chen L, Zhang Y (2010) Fabrication of mesoporous silica-coated CNTs and application in size-selective protein separation. J Mater Chem 20:5835–5842
Zhang S, Zhang Y, Liu J, Xu Q, Xiao H, Wang X, Xu H, Zhou J (2013) Thiol modified Fe3O4@SiO2 as a robust, high effective, and recycling magnetic sorbent for mercury removal. Chem Eng J 226:30–38
Zhanga F, Nriagu JO, Itoh H (2005) Mercury removal from water using activated carbons derived from organic sewage sludge. Water Res 39:389–395
Zhu J, Yang J, Deng B (2009) Enhanced mercury ion adsorption by amine-modified activated carbon. J Hazard Mater 166:866–872
Acknowledgments
The author would like to acknowledge the support provided by the Deanship of Scientific Research (DSR) at King Fahd University of Petroleum & Minerals (KFUPM) for funding this work through project No. JF121009.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Saleh, T.A. Isotherm, kinetic, and thermodynamic studies on Hg(II) adsorption from aqueous solution by silica- multiwall carbon nanotubes. Environ Sci Pollut Res 22, 16721–16731 (2015). https://doi.org/10.1007/s11356-015-4866-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4866-z