Abstract
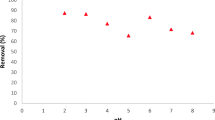
Our aim was to test how MWCNTs can be used as a new adsorbent for mercury(II). Multi-walled carbon nanotubes (MWCNTs) have been used for removal of mercury from aqueous solutions. Mercury removal from aqueous solutions by batch adsorption was investigated. Equilibrium isotherms, such as Freundlich, Langmuir, Temkin, Harkins-Jura, were tested. Kinetic studies based on Lagergren first-order, pseudo-second-order and Elovich rate expressions were done. The batch experiments were conducted at three different temperatures (17, 27 and 37 °C) and different pHs of the initial solution. Error function analysis shows that mercury(II) removal obeys pseudo-second order kinetics and Freundlich isotherm equation. Finally, the effects of solution pH and temperature on the adsorption were studied.
Similar content being viewed by others
References
N. E. Okoronkwo and J. C. Igwe, African J. Biotechnol., 6, 335 (2007).
X. Yang, Y. Zhuo, Y. Duan, L. Chen, L. Yang, L. Zhang, Y. Jiang and X. Xu, Korean J. Chem. Eng., 24, 711 (2007).
J. U. Kennedy and N. Sathyamurthy, J. Hazard. Mater., 142, 165 (2007).
E. Uedee, P. Ramakul, U. Pancharoen and A.W. Lothongkum, Korean J. Chem. Eng., 25, 1486 (2008).
E. S. Shumate and W.G. Strandberg, Comprehensive Biotechnology, 13, 235 (1985).
H. Eccles, J. Chem. Technol. Biotechnol., 49, 330 (1990).
L. E. Macaskie, J. Chem. Technol. Biotechnol., 49, 330 (1990).
M. Tsezos and A. A. Dutchmann, J. Chem. Technol. Biotechnol., 53, 1 (1992).
V. Tare, S. Chaudhari and M. Jawed, Water Sci. Technol., 26, 237 (1992).
D. Leppert, Mining Eng., 42, 604 (1990).
F. Cadena, R. Rizvi and R.W. Peters, In Hazardous and Industrial Wastes, Proceedings of the Twenty-Second Mid-Atlantic Industrial Waste Conference, Drexel University, 77 (1990).
M.K. Sreedhar, A. Madhukumar and T. S. Anirudhan, Indian J. Eng. Mater. Sci., 6, 279 (1999).
M. R. Mostafa, Adsorption Sci. Technol., 15, 551 (1997).
K. Kadirvalu, M. Kavipriya, C. Karthika, N. Vennilamani and S. Pattabhi, Carbon J., 42, 745 (2004).
I. Uzun and F. Güzel, Turk J. Chem., 24, 291 (2000).
S. Iijima, Helical Microtubules of Graphitic Carbon. Nature, 354, 56 (1991).
S. Iijima, Carbon Nanotubes: Past, Present and Future. Physica B, 323, 1 (2002).
W. Chung-His, Colloid Interf. Sci. J., 311, 338 (2007).
Ch. Lu and Hu. Chiu, Chem. Eng. Sci. J., 61, 1138 (2006).
B. Tawabini, S. Al-Khaldi, M. Atieh and M. Khaled, Water Sci. Technol.: A Journal of the International Association on Water Pollution Research, 61, 591 (2010).
G. Limousin and J. P. Gaudet, Appl. Geochem., 22, 249 (2007).
A. Shafaei and F. Zokaee Ashtiani, Chem. Eng. J., 133, 311 (2007).
A. Gunay, Adsorption equilibrium and kinetics, J. Hazard. Mater., 145, 221 (2007).
S. J. Allen and G. McKay, J. Colloid Interf. Sci., 280, 322 (2004).
Y. Ho, Scientometrics J., 59, 171 (2004).
Y. S. Ho and G. McKay, Trans. IChemE., 76, 332 (1998).
Y. S. Ho, J. Hazard. Mater., 136, 681 (2006).
W. Chung-His, J. Hazard. Mater., 144, 93 (2007).
C. Chen and X. Li, Colloids Surfaces A: Physicochem. Eng. Aspects., 302, 449 (2007).
R. H. Perry and D. Green, Perry’s Chem. Eng Handbook, 6th Ed., McGraw-Hill, New York (1984).
D. Touaibia and B. Benayada, Desalination, 186, 75 (2005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shadbad, M.J., Mohebbi, A. & Soltani, A. Mercury(II) removal from aqueous solutions by adsorption on multi-walled carbon nanotubes. Korean J. Chem. Eng. 28, 1029–1034 (2011). https://doi.org/10.1007/s11814-010-0463-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-010-0463-5