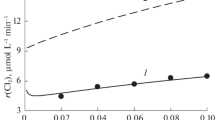
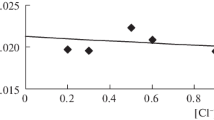
Abstract
The experimental investigation of chloride ion oxidation under the action of ozone and ultraviolet radiation with wavelength 254 nm in the bulk of acid aqueous solution at pH 0–2 has been performed. Processes of chloride oxidation in these conditions are the same as the chemical reactions in the system O3 – OH – Cl−(aq). Despite its importance in the environment and for ozone-based water treatment, this reaction system has not been previously investigated in the bulk solution. The end products are chlorate ion ClO3 − and molecular chlorine Cl2. The ions of trivalent iron have been shown to be catalysts of Cl− oxidation. The dependencies of the products formation rates on the concentrations of O3 and H+ have been studied. The chemical mechanism of Cl− oxidation and Cl2 emission and ClO3 − formation has been proposed. According to the mechanism, the dominant primary process of chloride oxidation represents the complex interaction with hydroxyl radical OH with the formation of Cl2 − anion-radical intermediate. OH radical is generated on ozone photolysis in aqueous solution. The key subsequent processes are the reactions Cl2 − + O3 → ClO + O2 + Cl− and ClO + H2O2 → HOCl + HO2. Until the present time, they have not been taken into consideration on mechanistic description and modelling of Cl− oxidation. The final products are formed via the reactions 2ClO → Cl2O2, Cl2O2 + H2O → 2H+ + Cl− + ClO3 − and HOCl + H+ + Cl− ⇄ H2O + Cl2. Some portion of chloride is oxidized directly by O3 molecule with the formation of molecular chlorine in the end.






Similar content being viewed by others
Notes
The Hatta number has been calculated with the formula Ha = \( \sqrt{D_{O3}{k}_{1,O3}/{k}_L} \) (Beltran 2004), where D O3 is ozone diffusion coefficient in aqueous solution, D O3 = 2 × 10−9 m2 s−1 (Beltran 2004), k L is the mass transfer coefficient, in the analogous reactors k L = 3 × 10−4 m s−1 (Benbelkacem et al. 2003; Khudoshin et al. 2008), k 1, O3 is the apparent pseudo-first-order rate constant of ozone reactions, in our experimental conditions k 1, O3 < 0.1 s−1. If Ha < 0.3, then ozone reactions are characterized as “slow”, and reactions in the interface film can be neglected compared with the reactions in the bulk of the liquid (Benbelkacem et al. 2003; Charpentier 1981; Sotelo et al. 1989).
Note that Fig. 5 in (Levanov et al. 2003a) presents the values of the apparent rate constant k app, L mol−1 min−1, of the thermal reaction between O3 and Cl− in aqueous solution, which is connected with the chlorine emission rate by the relation (dnCl2/dt)/V liq = k app∙[Cl−]∙HO3∙C(O3)∙1000/48, where [Cl−] = 1 M is chloride ion concentration in reaction solution, H O3 = 0.24 is dimensionless Henry’s constant of ozone (Levanov et al. 2003a), and C(O3), g/m3, is ozone concentration in the gas flow at the inlet to the reactor.
The photolysis primary stage (R1) was approximated as a reaction of first kinetic order with respect to ozone. Its rate constant was calculated with the formula k 1 = φ254 · N Φ · ε254 · ln10/(60 · NA), s−1, where φ254 = 0.55 (Reisz et al. 2003) is the primary quantum yield, N Φ = (4.34 ± 0.06) × 1020 photons L−1 min−1 is the rate of UV photons absorption by the reaction solution in the experiments of this work, and ε254 = 3000 L mol−1 cm−1 is the molar absorptivity of aqueous ozone solution, on the basis of (Bader and Hoigné 1982; Forni et al. 1982; Gilbert and Hoigne 1983; Hart et al. 1983; Hoigné 1998; Hoigne and Bader 1976; Kilpatrick et al. 1956; Taube 1957; von Sonntag and von Gunten 2012).
The rate constant k 4 was obtained with the use of ozone Henry’s law constant H O3 = 0.24 (Levanov et al. 2003a). If Henry’s law constant H O3 = 0.16 is used in the kinetic calculations, then the constant k 4 is recalculated correspondingly.
It is worth noting that the formation of chlorate ion on interaction of O3 with Cl−(aq) does not contradict the possibility of chlorate reduction with chloride in strong acid media (Gordon et al. 1972; Greenwood and Earnshaw 1997; Schmitz 2000),
ClO3 − + 5Cl− + 6H+ → 3Cl2 + 3H2O and ClO3 − + Cl− + 2H+ → ½Cl2 + ClO2 + H2O.
Quantitative estimates based on the kinetic data (Crisci and Lenzi 1971; Deshwal and Lee 2004; Hong et al. 1967; Sant'Anna et al. 2012) show that under the conditions of our experiments at pH 0–2, the rate of chlorate disappearance owing to these reactions is negligibly small.
Notice that the set of reactions (R1–R18) and the scheme of Fig. 6 account for the formation of only main products Cl2 and ClO3 −. The formation of an important minor product perchlorate ion is not described by the reactions and the scheme (and has not been investigated in this work), although it may well take place under the conditions of our experiments.
References
Adam LC, Fábián I, Suzuki K, Gordon G (1992) Hypochlorous acid decomposition in the pH 5–8 region. Inorg Chem 31:3534–3541
Alekseev VN (1969) Quantitative analysis. Mir Publishers, Moscow
Anderson A, Sun TS (1970) Raman spectra of molecular crystals I. Chlorine, bromine, and iodine. Chem Phys Lett 6:611–616. doi:10.1016/0009-2614(70)85239-3
Atkinson R et al (2007) Evaluated kinetic and photochemical data for atmospheric chemistry: volume III—gas phase reactions of inorganic halogens. Atmos Chem Phys 7:981–1191. doi:10.5194/acp-7-981-2007
Bader H, Hoigné J (1982) Colorimetric method for the measurement of aqueous ozone based on the decolorization of indigo derivatives. In: Masschelein WJ (ed) Ozonation manual for water and wastewater treatment. John Wiley and Sons, Chichester, UK, pp 169–172
Barb WG, Baxendale JH, George P, Hargrave KR (1949) Reactions of ferrous and ferric ions with hydrogen peroxide. Nature 163:692–694. doi:10.1038/163692a0
Barb WG, Baxendale JH, George P, Hargrave KR (1951a) Reactions of ferrous and ferric ions with hydrogen peroxide Part I.—The ferrous ion reaction. Trans Faraday Soc 47:462–500. doi:10.1039/TF9514700462
Barb WG, Baxendale JH, George P, Hargrave KR (1951b) Reactions of ferrous and ferric ions with hydrogen peroxide Part II.—The ferric ion reaction. Trans Faraday Soc 47:591–616. doi:10.1039/TF9514700591
Bartlett WP, Margerum DW (1999) Temperature dependencies of the Henry's Law constant and the aqueous phase dissociation constant of bromine chloride. Env Sci Technol 33:3410–3414. doi:10.1021/es990300k
Bauer D, D'Ottone L, Hynes AJ (2000) O 1D quantum yields from O3 photolysis in the near UV region between 305 and 375 nm. Phys Chem Chem Phys 2:1421–1424. doi:10.1039/B000159G
Baxendale JH, Wilson JA (1957) The photolysis of hydrogen peroxide at high light intensities. Trans Faraday Soc 53:344–356
Beach SD, Smith IWM, Tuckett RP (2002) Rate constants for the reaction of Cl atoms with O3 at temperatures from 298 to 184 K. In J Chem Kinet 34:104–109. doi:10.1002/kin.10033
Beltran FJ (2004) Ozone reaction kinetics for water and wastewater systems. . lewis publishers, CRC press LLC, boca raton, Florida
Benbelkacem H, Cano H, Mathe S, Debellefontaine H (2003) Maleic acid ozonation: reactor modeling and rate constants determination ozone. Sci Eng 25:13–24. doi:10.1080/713610647
Biedenkapp D, Hartshorn LG, Bair EJ (1970) The O (1D) + H2O reaction. Chem Phys Lett 5:379–381. doi:10.1016/0009-2614(70)85172-7
Bielski BHJ (1993) A pulse-radiolysis study of the reaction of ozone with cl2- radical-anion in aqueous-solutions. Radiat Phys Chem 41:527–530
Bjergbakke E, Navaratnam S, Parsons BJ, Swallow AJ (1981) Reaction between HO2 and chlorine IN aqueous solution. J Am Chem Soc 103:5926–5928. doi:10.1021/ja00409a059
Bräuer P, Tilgner A, Wolke R, Herrmann H (2013) Mechanism development and modelling of tropospheric multiphase halogen chemistry: the CAPRAM halogen module 2.0 (HM2). J Atmos Chem 70:19–52. doi:10.1007/s10874-013-9249-6
Broek VD (1862) Ueber die zersetzung der chlorwasserstoffsäure durch ozone (notizen). J Prakt Chem 86:317–318. doi:10.1002/prac.18620860142
Buxton GV, Bydder M, Salmon GA (1998) Reactivity of chlorine atoms in aqueous solution. Part 1. the equilibrium Cl• + Cl– = Cl2. J Chem Soc Faraday Trans 94:653–657. doi:10.1039/a707377a
Buxton GV, Subhani MS (1972a) Radiation chemistry and photochemistry of oxychlorine ions. Part 1. radiolysis of aqueous solutions of hypochlorite and chlorite ions. J Chem Soc Faraday Trans 1(68):947–957
Buxton GV, Subhani MS (1972b) Radiation chemistry and photochemistry of oxychlorine ions. Part 2. Photodecomposition of aqueous solutions of hypochlorite ions. J Chem Soc Faraday Trans 1(68):958–969
Cahill JE, Leroi GE (1969) Raman spectra of solid chlorine and bromine. J Chem Phys 51:4514–4519. doi:10.1063/1.1671821
Caldin EF (2001) The mechanisms of fast reactions in solution. Ios Press, Amsterdam
Charpentier J-C (1981) Mass-transfer rates in gas–liquid absorbers and reactors. In: Drew TB, Cokelet GR, Hoopes JW, Vermeulen T (eds) Advances in chemical engineering, vol.11, vol 11. Academic Pres, New York, pp 1–133. doi:10.1016/S0065-2377(08)60025-3
Connick RE (1947) The interaction of hydrogen peroxide and hypochlorous acid in acidic solutions containing chloride ion. J Am Chem Soc 69:1509–1514. doi:10.1021/ja01198a074
Crisci P, Lenzi F (1971) The kinetics and mechanism of the chloride–chlorate reaction in moderately concentrated solutions of HClO4 and H2SO4 at 25 °C. Can J Chem 49:2552–2562. doi:10.1139/v71-421
Dasgupta PK, Martinelango PK, Jackson WA, Anderson TA, Tian K, Tock RW, Rajagopalan S (2005) The origin of naturally occurring perchlorate: the role of atmospheric processes. Env Sci Tech 39:1569–1575
Deshwal BR, Lee HK (2004) Kinetics and mechanism of chloride based chlorine dioxide generation process from acidic sodium chlorate. J Hazard Mater 108:173–182. doi:10.1016/j.jhazmat.2003.12.006
Duverney MR (1962) Rayons restants infrarouges, de monocristaux de chlorate et de bromate de sodium. C R Acad Sci 254:1954–1956
Emmenegger F, Gordon G (1967) The rapid interaction between sodium chlorite and dissolved chlorine. Inorg Chem 6:633–635. doi:10.1021/ic50049a048
Fabian I, Gordon G (1992) Iron (III)-catalyzed decomposition of the chlorite ion: an inorganic application of the quenched stopped-flow method. Inorg Chem 31:2144–2150. doi:10.1021/ic00037a030
Forni L, Bahnemann D, Hart EJ (1982) Mechanism of the hydroxide ion-initiated decomposition of ozone in aqueous solution. J Phys Chem 86:255–259. doi:10.1021/j100391a025
Gilbert E, Hoigne J (1983) Messung von ozon in wasserwerken vergleich der dpd- und indigo-methode gas- und wasserfach. Wasser, Abwasser 124:527–531
Goldstein S, Aschengrau D, Diamant Y, Rabani J (2007) Photolysis of aqueous H2O2: quantum yield and applications for polychromatic UV actinometry in photoreactors env. Sci Technol 41:7486–7490. doi:10.1021/es071379t
Gordon G, Kieffer RG, Rosenblatt DH (1972) The chemistry of chlorine dioxide. In: Lippard SJ (ed) Progress in inorganic chemistry, vol 15, vol 15. Wiley, NewYork, pp 201–286. doi:10.1002/9780470166161.ch3
Gordon G, Tachiyashiki S (1991) Kinetics and mechanism of formation of chlorate ion from the hypochlorous acid/chlorite ion reaction at pH 6–10. Envirol Sci Technol 25:468–474. doi:10.1021/es00015a014
Greenwood NN, Earnshaw A (1997) Chemistry of the elements, Second Editionth edn. Butterworth-Heinemann, Oxford. doi:10.1016/B978-0-7506-3365-9.50003-1
Hart EJ, Sehested K, Holcman J (1983) Molar absorptivities of ultraviolet and visible bands of ozone in aqueous solutions. Anal Chem 55:46–49. doi:10.1021/ac00252a015
Hatta S (1932) Technological Reports of Tohoku University 10:613–662
Held AM, Halko DJ, Hurst JK (1978) Mechanisms of chlorine oxidation of hydrogen-peroxide. J Am Chem Soc 100:5732–5740. doi:10.1021/ja00486a025
Hoigné J (1998) Chemistry of Aqueous Ozone and Transformation of Pollutants by Ozonation and Advanced Oxidation Processes. In: Hrubec J (ed) The Handbook of Environmental Chemistry. Vol. 5, Part C. Quality and Treatment of Drinking Water II, vol 5 / 5C. The Handbook of Environmental Chemistry. Springer Verlag, Berlin - Heidelberg, pp 83–141. doi: 10.1007/978-3-540-68089-5_5
Hoigne J, Bader H (1976) Role of hydroxyl radical reactions in ozonation processes in aqueous solutions. Water Res 10:377–386
Hoigné J, Bader H, Haag WR, Staehelin J (1985) Rate constants of reactions of ozone with organic and inorganic compounds in water. III. Inorg Comp Rad Water Res 19:993–1004
Hollenberg JL, Dows DA, Spectrochim A (1960) Vibrational spectra of sodium chlorate. Spectrochim Acta 16:1155–1164. doi:10.1016/0371-1951(60)80220-2
Hong CC, Lenzi F, Rapson WH (1967) The kinetics and mechanism of the chloride-chlorate reaction. Can J Chem Eng 45:349–355. doi:10.1002/cjce.5450450605
Hong CC, Rapson WH (1968) Kinetics of disproportionation of chlorous acid. Can J Chem 46:2053–2060. doi:10.1139/v68-335
Hunt JP, Taube H (1952) The photochemical decomposition of hydrogen peroxide quantum yields, tracer and fractionation effects. J Am Chem Soc 74:5999–6002. doi:10.1021/ja01143a052
Ianni JC (2006) Kintecus , Windows Version 3.9, www.kintecus.com.
Jayson GG, Parsons BJ, Swallow AJ (1973) Some simple, highly reactive, inorganic chlorine derivatives in aqueous-solution. their formation using pulses of radiation and their role in mechanism of fricke dosimeter. J Chem Soc Faraday Trans I:1597–1607
Jia Z, Margerum DW, Francisco JS (2000) General-acid-catalyzed reactions of hypochlorous acid and acetyl hypochlorite with chlorite ion. Inorg Chem 39:2614–2620. doi:10.1021/ic991486r
Kang N, Jackson WA, Dasgupta PK, Anderson TA (2008) Perchlorate production by ozone oxidation of chloride in aqueous and dry systems. Sci Total Environ 405:301–309
Khudoshin AG, Mitrofanova AN, Lunin VV (2008) Kinetics and mechanism of the reactions of ozone with guaiacol, veratrol, and veratrol derivatives. Russ Chem Bull 57:283–288. doi:10.1007/s11172-008-0043-6
Kieffer RG, Gordon G (1968a) Disproportionation of chlorous acid. I. Stoichiometry Inorg Chem 7:235–239. doi:10.1021/ic50060a013
Kieffer RG, Gordon G (1968b) Disproportionation of chlorous acid. II. Kinet Inorg Chem 7:239–244. doi:10.1021/ic50060a014
Kilpatrick ML, Herrick CC, Kilpatrick M (1956) The decomposition of ozone in aqueous solution. J Am Chem Soc 78:1784–1789. doi:10.1021/ja01590a003
Kiwi J, Lopez A, Nadtochenko V (2000) Mechanism and kinetics of the oh-radical intervention during fenton oxidation in the presence of a significant amount of radical scavenger (cl-). Environ Sci Technol 34:2162–2168. doi:10.1021/es991406i
Klaning UK, Wolff T (1985) Laser flash photolysis of hclo, clo−, hbro, and bro− in aqueous solution reactions of cl- and br-atoms. Ber Bunsenges Phys Chem 89:243–245
Knipping EM, Dabdub D (2002) Modeling Cl2 formation from aqueous NaCl particles: evidence for interfacial reactions and importance of Cl2 decomposition in alkaline solution. J Geophys Res 107:4360. doi:10.1029/2001jd000867
Knipping EM et al (2000) Experiments and simulations of ion-enhanced interfacial chemistry on aqueous nacl aerosols. Science 288:301–306. doi:10.1126/science.288.5464.301
Koppenol WH, Stanbury DM, Bounds PL (2010) Electrode potentials of partially reduced oxygen species, from dioxygen to water. Free Radic Biol Med 49:317–322. doi:10.1016/j.freeradbiomed.2010.04.011
Langford CH, Carey JH (1975) The charge transfer photochemistry of the hexaaquoiron (iii) ion, the chloropentaaquoiron (iii) ion, and the μ-dihydroxo dimer explored with tert-butyl alcohol scavenging. Can J Chem 53:2430–2435. doi:10.1139/v75-344
Laskin A, Wang H, Robertson WH, Cowin JP, Ezell MJ, Finlayson-Pitts BJ (2006) A new approach to determining gas-particle reaction probabilities and application to the heterogeneous reaction of deliquesced sodium chloride particles with gas-phase hydroxyl radicals. J Phys Chem A 110:10619–10627. doi:10.1021/jp063263+
Levanov AV, Antipenko EE, Lunin VV (2012a) Primary stage of the reaction between ozone and chloride ions in aqueous solution: can chloride ion oxidation by ozone proceed via electron transfer mechanism? Russ J Phys Chem A 86:584–589. doi:10.1134/S0036024412040164
Levanov AV, Antipenko EE, Lunin VV (2012b) Primary stage of the reaction between ozone and chloride ions in aqueous solution: oxidation of chloride ions with ozone through the mechanism of oxygen atom transfer. Russ J Phys Chem A 86:519–522. doi:10.1134/s0036024412030193
Levanov AV, Kuskov IV, Antipenko EE, Lunin VV (2006a) The kinetics of reaction between permanganate and chlorine ions in acid solutions. Russ J Phys Chem 80:726–731. doi:10.1134/s0036024406050104
Levanov AV, Kuskov IV, Antipenko EE, Lunin VV (2006b) The oxidation of chlorine ions under the joint action of ozone and permanganate ions. Russ J Phys Chem 80:556–561. doi:10.1134/s0036024406040121
Levanov AV, Kuskov IV, Antipenko EE, Lunin VV (2008) The solubility of ozone and kinetics of its chemical reactions in aqueous solutions of sodium chloride. Russ J Phys Chem A 82:2045–2050. doi:10.1134/s0036024408120133
Levanov AV, Kuskov IV, Antipenko EE, Lunin VV (2012c) Stoichiometry and products of ozone reaction with chloride ion in an acidic medium. Russ J Phys Chem A 86:757–762. doi:10.1134/S0036024412050202
Levanov AV, Kuskov IV, Koiaidarova KB, Antipenko EI, Lunin VV (2006c) Interaction between ozone and the chloride ion in sulfuric acid solutions up to 6-M concentration. Kinet Catal 47:682–685. doi:10.1134/s0023158406050053
Levanov AV, Kuskov IV, Koiaidarova KB, Zosimov AV, Antipenko EE, Lunin VV (2005) Catalysis of the reaction of ozone with chloride ions by metal ions in an acidic medium. Kinet Catal 46:138–143. doi:10.1007/s10975-005-0021-z
Levanov AV, Kuskov IV, Zosimov AV, Antipenko EE, Lunin VV (2003a) Acid catalysis in reaction of ozone with chloride ions. Kinet Catal 44:740–746. doi:10.1023/B:KICA.0000009047.90252.2d
Levanov AV, Kuskov IV, Zosimov AV, Antipenko EE, Lunin VV (2003b) Photometric determination of chlorine in a gas flow in the presence of ozone. J Anal Chem 58:439–441. doi:10.1023/A:1024069912606
Levanov AV, Sakharov DV, Dashkova AV, Antipenko EE, Lunin VV (2011) Synthesis of hydrogen polyoxides H2O4 and H2O3 and their characterization by Raman spectroscopy. Eur J Inorg Chem 2011:5144–5150. doi:10.1002/ejic.201100767
Liu Q, Margerum DW (2001) Equilibrium and kinetics of bromine chloride hydrolysis. Environ Sci Technol 35:1127–1133
Makower B, Bray WC (1933) The rate of oxidation of hydrogen peroxide by chlorine in the presence of hydrochloric acid. J Am Chem Soc 55:4765–4776. doi:10.1021/ja01339a006
Maric D, Burrows JP, Meller R, Moortgat GK (1993) A study of the UV-visible absorption spectrum of molecular chlorine. J Photochem Photobiol A Chem 70:205–214
Mialocq JC, Barat F, Gilles L, Hickel B, Lesigne B (1973) Flash photolysis of chlorine dioxide in aqueous solution. J Phys Chem 77:742–749. doi:10.1021/j100625a003
Miller FA, Carlson GL, Bentley FF, Jones WH (1960) Infrared spectra of inorganic ions in the cesium bromide region (700–300 cm−1). Spectrochim Acta 16:135–235. doi:10.1016/0371-1951(60)80077-X
Miller FA, Wilkins CH (1952) Infrared spectra and characteristic frequencies of inorganic ions. Anal Chem 24:1253–1294. doi:10.1021/ac60068a007
Nadtochenko VA, Kiwi J (1998) Photolysis of feoh2+ and fecl2+ in aqueous solution. Photodissociation Kinetics and Quantum Yields Inorg Chem 37:5233–5238. doi:10.1021/ic9804723
Naumov S, von Sonntag C (2011) Standard gibbs free energies of reactions of ozone with free radicals in aqueous solution: quantum-chemical calculations. Environ Sci Technol 45:9195–9204. doi:10.1021/es2018658
Nissenson P, Thomas JL, Finlayson-Pitts BJ, Dabdub D (2008) Sensitivity and uncertainty analysis of the mechanism of gas-phase chlorine production from NaCl aerosols in the MAGIC model. Atmos Environ 42:6934–6941. doi:10.1016/j.atmosenv.2008.04.041
Oum KW, Lakin MJ, DeHaan DO, Brauers T, Finlayson-Pitts BJ (1998) Formation of molecular chlorine from the photolysis of ozone and aqueous sea-salt particles. Science 279:74–76. doi:10.1126/science.279.5347.74
Peintler G, Nagypal I, Epstein IR (1990) Systematic design of chemical oscillators. 60. kinetics and mechanism of the reaction between chlorite ion and hypochlorous acid. J Phys Chem 94:2954–2958. doi:10.1021/j100370a040
Pignatello JJ, Oliveros E, MacKay A (2006) Advanced Oxidation Processes for Organic Contaminant Destruction Based on the Fenton Reaction and Related Chemistry Critical Reviews in Environmental Science and Technology 36:1–84 doi:10.1080/10643380500326564
Quiroga SL, Perissinotti LJ (2005) Reduced mechanism for the 366 nm chlorine dioxide photodecomposition in N2-saturated aqueous solutions. J Photochem Photobiol A Chemistry 171:59–67. doi:10.1016/j.jphotochem.2004.09.006
Rabai G, Orban M (1993) General model for the chlorite ion based chemical oscillators. J Phys Chem 97:5935–5939. doi:10.1021/j100124a026
Rao B, Anderson TA, Redder A, Jackson WA (2010) Perchlorate formation by ozone oxidation of aqueous chlorine / oxy-chlorine species: role of clxoy radicals. Env Sci Technol 44:2961–2967
Reisz E, Schmidt W, Schuchmann HP, von Sonntag C (2003) Photolysis of ozone in aqueous solutions in the presence of tertiary butanol. Environ Sci Technol 37:1941–1948. doi:10.1021/es0113100
Sander R et al (2011a) The atmospheric chemistry box model CAABA/MECCA-3.0. Geosci Model Dev 4:373–380. doi:10.5194/gmd-4-373-201
Sander SP et al. (2011) Chemical kinetics and photochemical data for use in atmospheric studies, evaluation no. 17. Pasadena: JPL Publication 10–6, Jet Propulsion Laboratory, 2011.
Sant'Anna RTP, Santos CMP, Silva GP, Ferreira RJR, Oliveira AP, Côrtes CES, Faria RB (2012) Kinetics and mechanism of chlorate-chloride reaction. J Braz Chem Soc 23:1543–1550
Schmitz G (2000) Kinetics of the halates-halides-halogens reactions; apparent differences and fundamental similarities. In: Ribnikar S, Anic S (eds) Proceedings of the 5th International conference on fundamental and applied aspects of physical chemistry. Society of Physical Chemists, Belgrade, pp 129–140
Smith GD, Molina LT, Molina MJ (2000) Temperature dependence of O (1D) quantum yields from the photolysis of ozone between 295 and 338 nm. J Phys Chem A 104:8916–8921. doi:10.1021/jp001006d
Sotelo JL, Beltrán FJ, Benitez FJ, Beltrán-Heredia J (1989) Henry's law constant for the ozone-water system. Water Res 23:1239–1246. doi:10.1016/0043-1354(89)90186-3
Stanbury DM (1989) Reduction potentials involving inorganic free radicals in aqueous solution. in advances in inorganic chemistry, vol 33. pp 69–138
Su F, Calvert JG, Lindley CR, Uselman WM, Shaw JH (1979) Fourier transform infrared kinetic study of hypochlorous acid and its absolute integrated infrared band intensities. J Phys Chem 83:912–920. doi:10.1021/j100471a006
Suzuki M, Yokoyama T, Ito M (1969) Raman spectrum and intermolecular forces of the chlorine crystal. J Chem Phys 50:3392–3398
Taniguchi N, Takahashi K, Matsumi Y (2000) Photodissociation of O3 around 309 nm. J Phys Chem A 104:8936–8944. doi:10.1021/jp001706i
Taube H (1957) Photochemical reactions of ozone in solution. Trans Farad Soc 53:656–665. doi:10.1039/TF9575300656
Taube H, Dodgen H (1949) Applications of radioactive chlorine to the study of the mechanisms of reactions involving changes in the oxidation state of chlorine. J Am Chem Soc 71:3330–3336. doi:10.1021/ja01178a016
Thomas JL, Jimenez-Aranda A, Finlayson-Pitts BJ, Dabdub D (2006) Gas-phase molecular halogen formation from nacl and nabr aerosols: when are interface reactions important? J Phys Chem A 110:1859–1867. doi:10.1021/jp054911c
Thomas JL, Stutz J, Lefer B, Huey LG, Toyota K, Dibb JE, von Glasow R (2011) Modeling chemistry in and above snow at Summit, Greenland—part 1: model description and results. Atmos Chem Phys 11:4899–4914. doi:10.5194/acp-11-4899-2011
von Sonntag C, von Gunten U (2012) Chemistry of ozone in water and wastewater treatment. From basic principles to applications. IWA Publishing, London
Wagman DD et al. (1982) The NBS tables of chemical thermodynamic properties. J. Phys. Chem. Ref. Data, 1982, 11, Supplement №2. National Bureau of Standards, Washington D. C.
Wang TX, Kelley MD, Cooper JN, Beckwith RC, Margerum DW (1994) Equilibrium, kinetic, and UV-spectral characteristics of aqueous bromine chloride, bromine, and chlorine species. Inorg Chem 33:5872–5878
Wang TX, Margerum DW (1994) Kinetics of reversible chlorine hydrolysis: temperature dependence and general-acid/base-assisted mechanisms. Inorg Chem 33:1050–1055. doi:10.1021/ic00084a014
Wayne RP (1987) The photochemistry of ozone. Atmos Environ 21:1683–1694. doi:10.1016/0004-6981(87)90107-7
Wilkinson F, Helman WP, Ross AB (1995) Rate constants for the decay and reactions of the lowest electronically excited singlet state of molecular oxygen in solution. An expanded and revised compilation. J Phys Chem Ref Data 24:663–677. doi:10.1063/1.555965
Yeatts LRB, Taube H (1949) The kinetics of the reaction of ozone and chloride ion in acid aqueous solution. J Am Chem Soc 71:4100–4105
Yu X-Y, Barker JR (2003) Hydrogen peroxide photolysis in acidic aqueous solutions containing chloride ions I. Chem Mech J Phys Chem A 107:1313–1324. doi:10.1021/jp0266648
Yu XY (2004) Critical evaluation of rate constants and equilibrium constants of hydrogen peroxide photolysis in acidic aqueous solutions containing chloride ions. J Phys Chem Ref Data 33:747–763. doi:10.1063/1.1695414
Acknowledgments
The authors acknowledge partial support from M.V. Lomonosov Moscow State University Program of Development.
Conflict of Interest
The authors declare no competing interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
Electronic Supporting Information available:
The experimental and calculated dependencies of chlorine emission and chlorate formation rates on concentrations of H+ in reaction solution and O3 in initial gases (Figs. S1-S4).
The effect of variation of volumetric mass transfer coefficient kLa on calculated rates of chlorine emission and chlorate formation (Fig. S5).
Optimized values of coefficients k18 and n18 as functions of Henry’s law constant of ozone HO3 and volumetric mass transfer coefficient kLa (Fig. S6).
Summary of the reactions included in the mechanism of photochemical chloride oxidation with ozone (Table S1).
Effect of addition to the reaction set (R1 – R17) of various reactions, and also processes of HO2 disappearance and OH generation, on the calculated rates of chlorine emission and chlorate formation. (Tables S2-S4).
ESM 1
(DOCX 234 kb)
Rights and permissions
About this article
Cite this article
Levanov, A.V., Isaykina, O.Y., Amirova, N.K. et al. Photochemical oxidation of chloride ion by ozone in acid aqueous solution. Environ Sci Pollut Res 22, 16554–16569 (2015). https://doi.org/10.1007/s11356-015-4832-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4832-9