Abstract
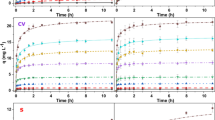
Discharge of dye-containing wastewater by the textile industry can adversely affect aquatic ecosystems and human health. Bioremoval is an alternative to industrial processes for detoxifying water contaminated with dyes. In this work, active and inactive biomass of the microalga Chlorella vulgaris was assayed for the ability to remove Congo Red (CR) dye from aqueous solutions. Through biosorption and biodegradation processes, Chlorella vulgaris was able to remove 83 and 58 % of dye at concentrations of 5 and 25 mg L−1, respectively. The maximum adsorption capacity at equilibrium was 200 mg g−1. The Langmuir model best described the experimental equilibrium data. The acute toxicity test (48 h) with two species of cladocerans indicated that the toxicity of the dye in the effluent was significantly decreased compared to the initial concentrations in the influent. Daphnia magna was the species less sensitive to dye (EC50 = 17.0 mg L−1), followed by Ceriodaphnia dubia (EC50 = 3.32 mg L−1). These results show that Chlorella vulgaris significantly reduced the dye concentration and toxicity. Therefore, this method may be a viable option for the treatment of this type of effluent.






Similar content being viewed by others
References
Acuner A, Dilek FB (2004) Treatment of Tectilon Yellow 2G by Chlorella vulgaris. Process Biochem 39:623–631
Aksu Z, Tezer S (2005) Biosorption of reactive dyes on the green algae Chlorella vulgaris. Process Biochem 40:1347–1361
Annadurai G, Juang RS, Lee DJ (2002) Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. J Hazard Mater B92:263–274
Bae JS, Freeman HS (2007) Aquatic toxicity evaluation of new Direct dyes to the Daphnia magna. Dyes Pigments 73:81–85
Basha S, Murthy ZVP (2007) Kinetic and equilibrium models for biosorption of Cr (VI) on chemically modified seaweed, Cystoseira indica. Process Biochem 42:1521–1529
Binupriya AR, Sathishkumar M, Swaminathan K, Kuz CS, Yun SE (2008) Comparative studies on removal of Congo Red by native and modified mycelial pellets of Trametes versicolor in various reactor modes. Bioresour Technol 99:1080–1088
Blaise C, Férard JF (2005) Small-scale freshwater toxicity investigations. Vol. 1–Toxicity test methods. Springer, Berlin, 422 p
Chowdhury S, Saha P (2010) Sea shell power as a new adsorbent to remove Basic Green 4 (Malachite Green) from aqueous solutions: equilibrium, kinetic and thermodynamic studies. Chem Eng J 164(1):168–177
Deniz F, Saygideger SD (2011) Removal of a hazardous azo dye (Basic Red 46) from aqueous solution by princess tree leaf. Desalination 268:6–11
El-Sheekh MM, Gharieb MM, Abou-El-Souod GW (2009) Biodegradation of dyes by some green algae and cyanobacteria. Int Biodeterior Biodegrad 63:699–704
Fu Y, Viraraghavan T (2002) Removal of Congo Red from an aqueous solution by fungus Aspergillus niger. Adv Environ Res 7:239–247
Giles CH, Smith D, Huitso A (1974) A general treatment and classification of the solute adsorption isotherms. I Theoretical. J Colloid Interface Sci 47:755–765
Golka K, Kopps S, Myslak WZ (2004) Carcinogenicity of azo colorants: influence of solubility and bioavailability. Toxicol Lett 15:203–210
Guendouz S, Khellaf N, Zerdaoui M, Ouchefoun M (2013) Biosorption of synthetic dyes (Direct Red 89 and Reactive Green 12) as an ecological refining step in textile effluent treatment. Environ Sci Pollut Res 20:3822–3829
Hanan HO (2008) Algal decolorization and degradation of monoazo and diazo dyes. Pak J Biol Sci 11(10):1310–1316
Hazrat A (2010) Biodegradation of synthetic dyes—a review. Water Air Soil Pollut 213:251–273
Hernández-Zamora M, Perales-Vela HV, Flores-Ortiz CM, Cañizares-Villanueva RO (2014) Physiological and biochemical responses of Chlorella vulgaris to Congo Red. Ecotoxicol Environ Saf 108:72–77
Jinqi L, Houtian L (1992) Degradation of azo dyes by algae. Environ Pollut 75:273–278
Kumar R, Ahmad R (2011) Biosorption of hazardous crystal violet dye from aqueous solution onto treated ginger waste (TGW). Desalination 265:112–118
Martínez-Jerónimo F, Espinosa-Chávez F, Villaseñor-Córdova R (2000) Effect of culture volume and adult density on the neonate production of Daphnia magna, as test organisms for aquatic toxicity test. Environ Toxicol 15:155–159
McKay G, El-Geundi MS, Nassar MM (1987) Equilibrium studies during the removal of dyestuffs from aqueous solutions using bagasse pith. Water Res 21:1523–1530
Meric S, Selçuk H, Belgiorno V (2005) Acute toxicity removal in textile finishing wastewater by Fenton’s oxidation, ozone and coagulation-floculation processes. Water Res 39(6):1147–1153
Mexican Standard NMX-AA-087-SCFI (2010) ANÁLISIS DE AGUA-Evaluación de Toxicidad Aguda con Daphnia magna Straus (Crustacea Cladocera)-Método de prueba, 44 pp
Michalak I, Chojnacka K (2010) The new application of biosorption properties of Enteromorpha prolifera. Appl Biochem Biotechnol 160:1540–1556
Namasivayam C, Prabha D, Kumar M (1998) Removal of Direct Red and Acid Brilliant Blue by adsorption on to banana pith. Bioresour Technol 64:77–79
Ong S, Uchiyama K, Inadama D, Ishida Y, Yamagiwa K (2010) Treatment of azo dye Acid Orange 7 containing wastewater using up-flow constructed wetland with and without supplementary aeration. Bioresour Technol 101:9049–9057
Pandey A, Singh P, Iyengar L (2007) Bacterial decolorization and degradation of azo dyes. Int Biodeterior Biodegrad 59:73–84
Safarikoval M, Pona BMR, Mosiniewicz-Szablewska E, Weyda F, Safarik I (2008) Dyes adsorption on magnetically modified Chlorella vulgaris cells. Fresenius Environ Bull 17:486–492
Saha PD, Chowdhury S, Mondal M, Sinha K (2012) Biosorption of Direct Red 28 (Congo Red) from aqueous solutions by eggshells: batch and column studies. Sep Sci Technol 47:112–123
Saratale RG, Saratale GD, Chang JS, Govindwar SP (2011) Bacterial decolorization and degradation of azo dyes: a review. J Taiwan Inst Chem Eng 42:138–157
Sponza DT, Isik M (2005) Toxicity and intermediates of C.I. Direct Red 28 dye through sequential anaerobic/aerobic treatment. Process Biochem 40:2735–2744
Stein JR (1973) Handbook of phycological methods. Culture methods and growth measurements. Cambridge University Press, London, pp 7–24
Stephan CE (1997) Methods for calculating an LC50. In: Mayer FL, Hamelink JL (ed) Aquatic toxicology and hazard evaluation. American Society for Testing and Materials (ASTM), Philadelphia Pennsylvania, pp. 65–84, 534.
Sudha M, Saranya A, Selvakumar G, Sivakumar N (2014) Microbial degradation of azo dyes: a review. Int J Curr Microbiol Appl Sci 3(2):670–690
USEPA (2002) Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms. 5ª ed. US Environmental Protection Agency, Office of Water (4303 T). N.W. Washington, DC 20460. EPA-821-R-02-012, 266 p
Versteeg DJ, Stalmans M, Dyer SD, Janssen C (1997) Ceriodaphnia and Daphnia: a comparison of their sensitivity to xenobiotics and utility as a test species. Chemosphere 34:869–892
Vijayaraghavan K, Padmesh TVN, Palanivelu K, Velan M (2006) Biosorption of nickel (II) ions onto Sargassum wightii: application of two-parameter and three-parameter isotherm models. J Hazard Mater B133:304–308
Wang XS, Chen JP (2009a) Biosorption of Congo Red from aqueous solution using wheat bran and rice bran: batch studies. Sep Sci Technol 44:1452–1466
Wang XS, Chen JP (2009b) Removal of the azo dye Congo Red from aqueous solutions by the marine alga Porphyra yezoensis Ueda. Clean 37(10):793–798
Acknowledgments
The authors thank the Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional for the support to develop this investigation. Hernández-Zamora M. received a post-graduate scholarship from Consejo Nacional de Ciencia y Tecnología (Grant no. 204491) for doctoral studies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Hernández-Zamora, M., Cristiani-Urbina, E., Martínez-Jerónimo, F. et al. Bioremoval of the azo dye Congo Red by the microalga Chlorella vulgaris . Environ Sci Pollut Res 22, 10811–10823 (2015). https://doi.org/10.1007/s11356-015-4277-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4277-1