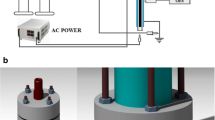
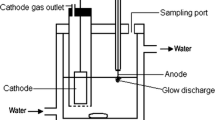
Abstract
Degradation of aqueous 3,4-dichloroaniline (3,4-DCA) was conducted in a novel dielectric barrier discharge (DBD) plasma reactor. The factors affecting the degradation efficiency of 3,4-DCA and the degradation mechanism of 3,4-DCA were investigated. The experimental results indicated that the degradation efficiency of 3,4-DCA increased with increasing input power intensity, and the degradation of 3,4-DCA by the novel DBD plasma reactor fitted pseudo-first-order kinetics. Higher degradation efficiency of 3,4-DCA was observed in acidic conditions. The degradation efficiency of 3,4-DCA, the removal rate of total organic carbon (TOC), and the detected Cl− increased dramatically with adding Fe2+ or Fe3+. Degradation of 3,4-DCA could be accelerated or inhibited in the presence of H2O2 depending on the dosage. Several degradation intermediates of 3,4-DCA such as 1,2-dichlorobenzene, 2-chloro-1,4-benzoquinone, 3,4-dichlorophenyl isocyanate, 2-chlorohydroquinone, 3,4-dichloronitrobenzene, and 3,4-dichlorophenol were identified by gas chromatography mass spectrometry (GC-MS) analysis. Based on the identification of aromatic intermediates, acetic acid, formic acid, oxalic acid, and Cl− released, a possible mineralization pathway of 3,4-DCA was proposed.












Similar content being viewed by others
References
Abdelmalek F, Ghezzar MR, Belhadj M, Addou A, Brisset JL (2006) Bleaching and degradation of textile dyes by nonthermal plasma process at atmospheric pressure. Ind Eng Chem Res 45:23–29
Alexander AB, Raynor CT, Wiggins DL, Robinson MK, Akpovo CC, Johnson JA (2011) Turbulence changes from magnetic fields in a stationary plasma. J Plasma Phys 77:537–545
Badawi N, Rønhede S, Olsson S, Kragelund BB, Johnsen AH, Jacobsen OS, Aamand J (2009) Metabolites of the phenylurea herbicides chlorotoluron, diuron, isoproturon and linuron produced by the soil fungus Mortierella sp. Environ Pollut 157:2806–2812
Behnajady MA, Modirshahla N, Ghanbary F (2007) A kinetic model for the decolorization of C.I. Acid Yellow 23 by Fenton process. J Hazard Mater 148:98–102
Beyerle-Pfnür R, Lay JP (1990) Adsorption and desorption of 3,4-dichloroaniline on soil. Chemosphere 21:1087–1094
Borràs N, Arias C, Oliver R, Brillas E (2013) Anodic oxidation, electro-Fenton and photoelectro-Fenton degradation of cyanazine using a boron-doped diamond anode and an oxygen-diffusion cathode. J Electroanal Chem 689:158–167
Breugelmans P, Leroy B, Bers K, Dejonghe W, Wattiez R, De Mot R, Springael D (2010) Proteomic study of linuron and 3,4-dichloroaniline degradation by Variovorax sp. WDL1: evidence for the involvement of an aniline dioxygenase-related multicomponent protein. Res Microbiol 161:208–218
Cagnoni D, Agostini F, Christen T, Parolini N, Stevanovic I, de Falco C (2013) Multiphysics simulation of corona discharge induced ionic wind. J Appl Phys 114:23301
Carrier M, Besson M, Guillard C, Gonze E (2009) Removal of herbicide diuron and thermal degradation products under Catalytic Wet Air Oxidation conditions. Appl Catal B Environ 91:275–283
Carvalho G, Marques R, Lopes AR, Faria C, Noronha JP, Oehmen A, Nunes OC, Reis MAM (2010) Biological treatment of propanil and 3,4-dichloroaniline: kinetic and microbiological characterisation. Water Res 44:4980–4991
Castillo JM, Nogales R, Romero E (2014) Biodegradation of 3,4 dichloroaniline by fungal isolated from the preconditioning phase of winery wastes subjected to vermicomposting. J Hazard Mater 267:119–127
Chen RZ, Pignatello JJ (1997) Role of quinone intermediates as electron shuttles in Fenton and photoassisted Fenton oxidation of aromatic compounds. Environ Sci Technol 31:2345–2349
Chen GL, Zhou MY, Chen SH, Chen WX (2009) The different effects of oxygen and air DBD plasma byproducts on the degradation of methyl violet 5BN. J Hazard Mater 172:786–791
Chen M, Jin LS, Liu YH, Guo XR, Chu JW (2014) Decomposition of NO in automobile exhaust by plasma-photocatalysis synergy. Environ Sci Pollut Res 21:1242–1247
Choi JH, Han I, Baik HK, Lee MH, Han DW, Park JC, Lee IS, Song KM, Lim YS (2006) Analysis of sterilization effect by pulsed dielectric barrier discharge. J Electrostat 64:17–22
Claver A, Ormad P, Rodriguez L, Ovelleiro JL (2006) Study of the presence of pesticides in surface waters in the Ebro river basin (Spain). Chemosphere 64:1437–1443
Dojcinovic BP, Roglic GM, Obradovic BM, Kuraica MM, Kostic MM, Nesic J, Manojlovic DD (2011) Decolorization of reactive textile dyes using water falling film dielectric barrier discharge. J Hazard Mater 192:763–771
Duan LJ, Shang KF, Lu N, Li J, Wu Y (2013) Study on the factors influencing phenol degradation in water by dielectric barrier discharge (DBD). J Phys Conf Ser 418:012129
Fancey KS (1995) An investigation into dissociative mechanisms in nitrogenous glow discharges by optical emission spectroscopy. Vacuum 46:695–700
Fava L, Orru MA, Crobe A, Caracciolo AB, Bottoni P, Funari E (2005) Pesticide metabolites as contaminants of groundwater resources: assessment of the leaching potential of endosulfan sulfate, 2,6-dichlorobenzoic acid, 3,4-dichloroaniline, 2.4-dichlorophenol and 4-chloro-2-methylphenol. Microchem J 79:207–211
Fava L, Orru MA, Businelli D, Scardala S, Funari E (2006) Leaching potential of some phenylureas and their main metabolites through laboratory studies. Environ Sci Pollut Res 13:386–391
Feng JW, Zheng Z, Sun YB, Luan JF, Wang Z, Wang LH, Feng JF (2008) Degradation of diuron in aqueous solution by dielectric barrier discharge. J Hazard Mater 154:1081–1089
Feng JW, Sun YB, Zhao DY, Zheng Z, Xu YW, Yang HF, Zhu HB, Zhou XX (2009a) Modeling of carbon monoxide removal by corona plasma. Plasma Sci Technol 11:598–603
Feng JW, Zheng Z, Luan JF, Li KQ, Wang LH (2009b) Gas-liquid hybrid discharge-induced degradation of diuron in aqueous solution. J Hazard Mater 164:838–846
Feng XL, Yan BH, Lu W, Jin Y, Cheng Y (2014) Visualization of coupled mass transfer and reaction in a gas-liquid dielectric barrier discharge reactor. Chem Eng J 245:47–55
Fernandez J, Bandara J, Lopez A, Buffat P, Kiwi J (1999) Photoassisted Fenton degradation of nonbiodegradable azo dye (Orange II) in Fe-free solutions mediated by cation transfer membranes. Langmuir 15:185–192
Franch MI, Ayllón JA, Peral J, Domènech X (2002) Photocatalytic degradation of short-chain organic diacids. Catal Today 76:221–233
Gandhi MS, Mok YS (2014) Non-thermal plasma-catalytic decomposition of volatile organic compounds using alumina supported metal oxide nanoparticles. Surf Coat Tech. doi:10.1016/j. surfcoat.2014.07.085
Gandhi MS, Ananth A, Mok YS, Song JI, Park KH (2013a) Time dependence of ethylene decomposition and byproducts formation in a continuous flow dielectric-packed plasma reactor. Chemosphere 91:685–691
Gandhi MS, Mok YS, Lee SB, Park H (2013b) Effect of various parameters for butane decomposition under ambient temperature in a dielectric barrier discharge non-thermal plasma reactor. J Taiwan Inst Chem E 44:786–794
Güçeri S, Fridman A (2007) Plasma assisted decontamination of biological and chemical agents. Springer, Netherlands
Guivan MM, Malinina AA, Brablec A (2011) Experimental and theoretical characterization of a multi-wavelength DBD-driven exciplex lamp operated with mercury bromide/rare gas mixtures. J Phys D Appl Phys 44:224012
Haag WR, Yao CCD (1992) Rate constants for reaction of hydroxyl radicals with several drinking water contaminants. Environ Sci Technol 26:1005–1013
Hong Y, Pan J, Lu N, Shang KF, Li J, Wu Y (2013) Low temperature air plasma jet generated by syringe needle-ring electrodes dielectric barrier discharge at atmospheric pressure. Thin Solid Films 548:470–474
Huang FM, Chen L, Wang HL, Yan ZC (2010) Analysis of the degradation mechanism of methylene blue by atmospheric pressure dielectric barrier discharge plasma. Chem Eng J 162:250–256
Huang FM, Chen L, Wang HL, Feng TZ, Yan ZC (2012) Degradation of methyl orange by atmospheric DBD plasma: Analysis of the degradation effects and degradation path. J Electrost 70:43–47
Jiang B, Zheng JT, Qiu S, Wu MB, Zhang QH, Yan ZF, Xue QZ (2014) Review on electrical discharge plasma technology for wastewater remediation. Chem Eng J 236:348–368
Joshi AA, Locke BR, Arce P, Finney WC (1995) Formation of hydroxyl radicals, hydrogen peroxide and aqueous electrons by pulsed streamer corona discharge in aqueous solution. J Hazard Mater 41:3–30
Karakas E, Begum A, Laroussi M (2008) A positive corona-based ion wind generator. IEEE T Plasma Sci 36:950–951
Kimura T, Yoshigoe T, Oda A (2003) Ozone production efficiency in atmospheric dielectric barrier discharge of oxygen/rare-gas mixture. Jpn J Appl Phys 142:5313–5314
Kogelschatz U (2003) Dielectric-barrier discharges: their history, discharge physics, and industrial applications. Plasma Chem Plasma P 23:1–46
Kurniawan TA, Lo WH, Chan GYS (2006) Radicals-catalyzed oxidation reactions for degradation of recalcitrant compounds from landfill leachate. Chem Eng J 125:35–57
Kusic H, Koprivanac N, Locke BR (2005) Decomposition of phenol by hybrid gas/liquid electrical discharge reactors with zeolite catalysts. J Hazard Mater 125:190–200
Lee HM, Chang MB, Wei TC (2004) Kinetic modeling of ozone generation via dielectric barrier discharges. Ozone Sci Eng 26:551–562
Li WM, Yin DQ, Zhou Y, Hu SQ, Wang LS (2003) 3,4-Dichloroaniline-induced oxidative stress in liver of crucian carp (Carassius auratus). Ecotox Environ Safe 56:251–255
Li JS, Wang XJ, Deng R, Pang ZH (2013) Comparison of degradation and decoloration on cigarette industry wastewater by ozone and advanced oxidation. Appl Mech Mater 295–298:1168–1172
Liu YJ, Jiang XZ (2005) Phenol degradation by a nonpulsed diaphragm glow discharge in an aqueous solution. Environ Sci Technol 39:8512–8517
Lodha B, Chaudhari S (2007) Optimization of Fenton-biological treatment scheme for the treatment of aqueous dye solutions. J Hazard Mater 148:459–466
López-Fernández JA, Peña-Eguiluz R, Mercado-Cabrera A, Jaramillo-Sierra B, López-Callejas R, Valencia-Alvarado R, Muñoz-Castro AE, Rodríguez-Méndez B, Barocio SR, de la Piedad-Beneitez A (2012) DBD reactor instrumentation for NOx degradation. Eur Phys J Appl Phys 57:10802
Lukes P, Locke BR (2005a) Degradation of substituted phenols in a hybrid gas-liquid electrical discharge reactor. Ind Eng Chem Res 44:2921–2930
Lukes P, Locke BR (2005b) Plasmachemical oxidation processes in a hybrid gas-liquid electrical discharge reactor. J Phys D Appl Phys 38:4074–4081
Lukes P, Appleton AT, Locke BR (2004) Hydrogen peroxide and ozone formation in hybrid gas-liquid electrical discharge reactors. IEEE T Ind Appl 40:60–67
Malato S, Cáceres J, Fernández-Alba AR, Piedra L, Hernando MD, Agüera A, Vial J (2003) Photocatalytic treatment of diuron by solar photocatalysis: evaluation of main intermediates and toxicity. Environ Sci Technol 37:2516–2524
Miichi T, Fujimoto T, Takeda T (2014) Decomposition of persistent organic compounds in water using pulsed discharge on water. Electr Eng Jpn 186:1–10
Mitsou K, Koulianou A, Lambropoulou D, Pappas P, Albanis T, Lekka M (2006) Growth rate effects, responses of antioxidant enzymes and metabolic fate of the herbicide Propanil in the aquatic plant Lemna minor. Chemosphere 62:275–284
Mok YS, Ham SW, Nam IS (1998) Mathematical analysis of positive pulsed corona discharge process employed for removal of nitrogen oxides. IEEE T Plasma Sci 26:1566–1574
Ndong ACA, Zouzou N, Benard N, Moreau E (2013) Geometrical optimization of a surface DBD powered by a nanosecond pulsed high voltage. J Electrostatat 71:246–253
Neyens E, Baeyens J (2003) A review of classic Fenton’s peroxidation as an advanced oxidation technique. J Hazard Mater 98:33–50
Ognier S, Iya-sou D, Fourmond C, Cavadias S (2009) Analysis of mechanisms at the plasma-liquid interface in a gas-liquid discharge reactor used for treatment of polluted water. Plasma Chem Plasma P 29:261–273
Oliveira FR, Erkens L, Fangueiro R, Souto AP (2012) Surface modification of banana fibers by DBD plasma treatment. Plasma Chem Plasma P 32:259–273
Ormad MP, Miguel N, Claver A, Matesanz JM, Ovelleiro JL (2008) Pesticides removal in the process of drinking water production. Chemosphere 71:97–106
Pacheco-Pacheco M, Pacheco-Sotelo J, Moreno-Saavedra H, Diaz-Gomez JA, Mercado-Cabrera A, Yousfi M (2007) DBD-corona discharge for degradation of toxic gases. Plasma Sci Technol 9:682–685
Padmini E, Miranda LR (2013) Nanocatalyst from sol-sol doping of TiO2 with Vanadium and Cerium and its application for 3,4 Dichloroaniline degradation using visible light. Chem Eng J 232:249–258
Pignatello JJ (1992) Dark and photoassisted iron(3+)-catalyzed degradation of chlorophenoxy herbicides by hydrogen peroxide. Environ Sci Technol 26:944–951
Planas C, Puig A, Rivera J, Caixach J (2006) Analysis of pesticides and metabolites in Spanish surface waters by isotope dilution gas chromatography/mass spectrometry with previous automated solid-phase extraction: Estimation of the uncertainty of the analytical results. J Chromatogr A 1131:242–52
Polcaro AM, Mascia M, Palmas S, Vacca A (2004) Electrochemical degradation of diuron and dichloroaniline at BDD electrode. Electrochim Acta 49:649–656
Sahni M, Locke BR (2006) The effects of reaction conditions on liquid-phase hydroxyl radical production in gas-liquid pulsed-electrical-discharge reactors. Plasma Process Polym 3:668–681
Saquib M, Abu Tariq M, Haque MM, Muneer M (2008) Photocatalytic degradation of disperse blue 1 using UV/TiO2/H2O2 process. J Environ Manag 88:300–306
Shimizu T, Iwafuchi Y, Morfill GE, Sato T (2011) Formation of thermal flow fields and chemical transport in air and water by atmospheric plasma. New J Phys 13:053025
Spinks JWT, Woods RJ (1976) An introduction to radiation chemistry. Wiley, New York
Sugiarto AT, Ito S, Ohshima T, Sato M, Skalny JD (2003) Oxidative decoloration of dyes by pulsed discharge plasma in water. J Electrostat 58:135–145
Sun Y, Pignatello JJ (1993) Photochemical reactions involved in the total mineralization of 2,4-D by iron(3+)/hydrogen peroxide/UV. Environ Sci Technol 27:304–310
Takayama M, Ebihara K, Stryczewska H, Ikegami T, Gyoutoku Y, Kubo K, Tachibana M (2006) Ozone generation by dielectric barrier discharge for soil sterilization. Thin Solid Films 506:396–399
Tendero C, Tixier C, Tristant P, Desmaison J, Leprince P (2006) Atmospheric pressure plasmas: a review. Spectrochim Acta B 61:2–30
Tizaoui C, Grima N, Hilal N (2011) Degradation of the antimicrobial triclocarban (TCC) with ozone. Chem Eng Process 50:637–643
Van Durme J, Dewulf J, Leys C, Van Langenhove H (2008) Combining non-thermal plasma with heterogeneous catalysis in waste gas treatment: a review. Appl Catal B Environ 78:324–333
Vatazhin AB, Likhter VA, Ulybyshev KE (2012) “Ion wind”, a gas-dynamic flow in the corona discharge, and its interaction with the external flow. Fluid Dynam+ 47:206–213
Venny, Gan SY, Ng NK (2012) Current status and prospects of Fenton oxidation for the decontamination of persistent organic pollutants (POPs) in soils. Chem Eng J 213:295–317
Walling CW (1975) Fenton’s reagent revisited. Accounts Chem Res 8:125–131
Wang HJ, Li J, Quan X (2006) Decoloration of azo dye by a multi-needle-to-plate high-voltage pulsed corona discharge system in water. J Electrostat 64:416–421
Wang SJ, Poon K, Cai ZW (2013) Biodegradation and removal of 3,4-dichloroaniline by Chlorella pyrenoidosa based on liquid chromatography-electrospray ionization-mass spectrometry. Environ Sci Pollut Res 20:552–557
Wei LS, Zhou JH, Wang ZH, Cen KF (2007) Theoretical analysis of ozone generation by pulsed dielectric barrier discharge in oxygen. J Plasma Phys 73:427–432
Wei LS, Yuan DK, Zhang YF, Hu ZJ, Dong GP (2013) The effect of inert gases on ozone generation using dielectric barrier discharge in dry air. Ozone Sci Eng 35:448–455
Yanallah K, Pontiga F, Castellanos A (2011) Numerical simulation of an oxygen-fed wire-to-cylinder negative corona discharge in the glow regime. J Phys D Appl Phys 44:055201
Yang GP, Zhao XK, Sun X, Lu XL (2005) Oxidative degradation of diethyl phthalate by photochemically-enhanced Fenton reaction. J Hazard Mater 126:112–118
Yao CCD, Haag WR (1991) Rate constants for direct reactions of ozone with several drinking water contaminants. Water Res 25:761–773
Zhang RB, Wu Y, Li GF, Wang NH (2004) Plasma induced degradation of Indigo Carmine by bipolar pulsed dielectric barrier discharge (DBD) in the water-air mixture. J Environ Sci (China) 16:808–812
Zhang Y, Zhou MH, Hao XL, Lei LC (2007) Degradation mechanisms of 4-chlorophenol in a novel gas-liquid hybrid discharge reactor by pulsed high voltage system with oxygen or nitrogen bubbling. Chemosphere 67:702–711
Zhu WP, Yang ZH, Wang L (1996) Application of ferrous-hydrogen peroxide for the treatment of H-acid manufacturing process wastewater. Water Res 30:2949–2954
Acknowledgments
This work was supported by the National Natural Science Foundation of China (51208163, 51108149), Open Fund of State Key Laboratory of Hydrology-Water Resources and Hydraulic Engineering, Hohai University (2013491211), and Open Topic of State Key Laboratory of Pollution Control and Resource Reuse, Nanjing University (PCRRF11014).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Angeles Blanco
Rights and permissions
About this article
Cite this article
Feng, J., Liu, R., Chen, P. et al. Degradation of aqueous 3,4-dichloroaniline by a novel dielectric barrier discharge plasma reactor. Environ Sci Pollut Res 22, 4447–4459 (2015). https://doi.org/10.1007/s11356-014-3690-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3690-1