Abstract
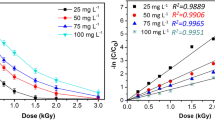
Radiolytic reactions of phenylureas were studied in detail with fenuron model compound in dilute aqueous solutions using pulse radiolysis for detection of the intermediates, gamma radiolysis with UV–Vis and HPLC-MS techniques for analysis of the final products. The kinetics of oxidation was followed by COD, TOC and toxicity measurements. During radiolysis of aerated solutions hydroxyl radical (•OH), eaq −, H• and O2 •−/HO2 • reactive intermediates are produced, the degradation of solute takes place practically entirely through •OH reactions. Therefore, the product distribution is similar to the distributions reported in other advanced oxidation processes with •OH as main reactant. •OH mainly reacts with the aromatic ring, forming cyclohexadienyl radical as an intermediate. This radical in pulse radiolysis has a wide absorption band in the 310–390 nm wavelength range with a maximum at 350 nm. Cyclohexadienyl radical reacts with dissolved O2 with a rate coefficient of ∼4 × 108 mol−1 dm3 s−1 forming peroxy radical. The latter may eliminate HO2 • giving phenols or undergoes fragmentation. The one-electron oxidant •OH on average induces more than two-electron oxidations. The toxicity first increases with absorbed dose, then decreases. This increase is partly due to phenols formed during the first degradation period.







Similar content being viewed by others
References
Acero JL, Benitez FJ, González M, Benitez R (2002) Kinetics of fenuron decomposition by single-chemical oxidants and combined systems. Ind Eng Chem Res 41:4225–4232
Amine-Khodja A, Boulkamh A, Boule P (2004) Photochemical behavior of phenylurea herbicides. Photochem Photobiol Sci 3:145–156
Brahmia O, Boulkamh A, Sehili T, Aguer J-P, Richard C (2002) Kinetics of photocatalytic decomposition of Fenuron over TiO2 in aqueous solution. Int J Photoenergy 4:85–89
Buxton G, Greenstock CL, Helman WP, Ross AB (1988) Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (•OH/O−) in aqueous solution. J Phys Chem Ref Data 17:513–886
Canle Lopez M, Fernandez MI, Rodrígez S, Santaballa JA, Steenken S, Vulliet E (2005) Mechanisms of direct and TiO2-photocatalised UV degradation of phenylurea herbicides. ChemPhysChem 6:2064–2074
Canle LM, Rodrígez S, Rodrígez Vazques LF, Santaballa JA, Steenken S (2001) First stages of photodegradation of the urea herbicides Fenuron, Monuron and Diuron. J Mol Struct 565–566:133–139
Dao YH, De Laat J (2011) Hydroxyl radical involvement in the decomposition of hydrogen peroxide by ferrous and ferric-nitrilotriacetate complexes at neutral pH. Water Res 45:3309–2317
Fang X, Pan X, Rahmann A, Schuchmann H-P, von Sonntag C (1995) Reversibility in the reaction of cyclohexadienyl radicals with oxygen in aqueous solution. Chem Eur J 1:423–429
Gallard H, De Laat J (2001) Kinetics of oxidation of chlorobenzenes and phenyl-ureas by Fe(II)/H2O2 and Fe(III)/H2O2. Evidence of reduction and oxidation reactions of intermediates by Fe(II) or Fe(III). Chemosphere 42:405–413
Gunatilleka AD, Poole CF (1999) Models for estimating the non-specific toxicity of organic compounds. Anal Chem 36:235–242
Homlok R, Takács E, Wojnárovits L (2013) Degradation of organic molecules in advanced oxidation processes: relation between chemical structure and degradability. Chemosphere 91:383–389
Kosaka K, Yamada H, Matsui S, Echigo S, Shishida K (1998) Comparison among the methods for hydrogen peroxide measurements to evaluate advanced oxidation processes: application of a spectrophotometric method using copper(II) ion and 2,9-dimethyl-1,10-phenanthroline. Environ Sci Technol 32:3821–3824
Mazellier P, Busset C, Delmont A, De Laat J (2007) A comparison of fenuron degradation by hydroxyl and carbonate radicals in aqueous solution. Water Res 41:4585–4594
Mvula E, Schuchmann MN, von Sonntag C (2001) Reactions of phenol-OH-adduct radicals. Phenoxyl radical formation by water elimination vs. oxidation by dioxygen. J Chem Soc Perkin Trans 2:264–268
Oturan MA, Edelahi MC, Oturan N, El Kacemi K, Aaron J-J (2010) Kinetics of oxidative degradation/mineralization pathways of the phenylurea herbicides diuron, monuron and fenuron in water during application of the electro-Fenton process. Appl Catal B Environ 97:82–89
Radiation processing: environmental applications (2007) International Atomic Energy Agency, Vienna, ISBN 92-D-100507-5
Richard C, Bengana S (1996) PH effect in the photocatalytic transformation of a phenyl-urea herbicide. Chemosphere 33:635–641
Salvestrini S, Di Cerbo P, Capasso S (2002) Kinetics of the chemical degradation of diuron. Chemosphere 48:69–73
Sarpal RS, Dogra SK (1987) Prototropism in aminophenols and anisidines: a reinvestigation. J Photochem 38:263–276
Singh TS, Gejji SP, Rao BSM, Mohan H, Mittal JP (2001) Radiation chemical oxidation of aniline derivatives. J Chem Soc Perkin Trans 2:1205–1211
Solar S, Solar W, Getoff N (1986) Resolved multisite OH-attack on aqueous aniline studied by pulse radiolysis. Radiat Phys Chem 28:229–234
Spinks JWT, Woods RJ (1990) An introduction to radiation chemistry, 3rd edn. Wiley-Interscience, New York
Takács E, Wojnárovits L, Dajka K (2000) Kinetics of the early stages of high-energy radiation initiated polymerization. Macromol Chem Phys 201:2170–2175
Villa S, Migliorati S, Monti GS, Vighi M (2012) Toxicity on the luminescent bacterium Vibrio fischeri (Beijerinck). II: response to complex mixtures of heterogeneous chemicals at low levels of individual components. Ecotoxicol Environ Saf 86:93–100
von Sonntag C, Schuchmann H-P (2001) Peroxyl radicals in aqueous solution. In: Alfassi ZB (ed) Peroxy-radicals. Wiley, Chichester, pp 173–274
Wojnárovits L, Takács E (2013) Structure dependence of the rate coefficients of hydroxyl radical + aromatic molecule reaction. Radiat Phys Chem 87:82–87
Yamasita S, Katsumura Y, Lin M, Muroya Y, Miyazaki T, Murakami T (2008) Water radiolysis with heavy ions of energies up to 28 GeV. 1. Measurements of primary g values as track segment yields. Radiat Phys Chem 77:439–446
Zhang J, Zheng Z, Zhao T, Zhao Y, Wang L, Zhong Y, Xu Y (2008) Radiation-induced reduction of diuron by gamma-ray irradiation. J Haz Mat 151:465–472
Zona R, Solar S (2003) Oxidation of 2,4-dichlorophenoxyacetic acid by ionizing radiation: degradation, detoxification and mineralization. Radiat Phys Chem 66:137–143
Acknowledgments
The authors thank the Hungarian Science Foundation (OTKA, NK 105802) and International Atomic Energy Agency (Contract No. 16485) for support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Roland Kallenborn
Rights and permissions
About this article
Cite this article
Kovács, K., Mile, V., Csay, T. et al. Hydroxyl radical-induced degradation of fenuron in pulse and gamma radiolysis: kinetics and product analysis. Environ Sci Pollut Res 21, 12693–12700 (2014). https://doi.org/10.1007/s11356-014-3197-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3197-9