Abstract
To investigate the spatial and seasonal variations of nitrous oxide (N2O) fluxes and understand the key controlling factors, we explored N2O fluxes and environmental variables in high marsh (HM), middle marsh (MM), low marsh (LM), and mudflat (MF) in the Yellow River estuary throughout a year. Fluxes of N2O differed significantly between sampling periods as well as between sampling positions. During all times of day and the seasons measured, N2O fluxes ranged from −0.0051 to 0.0805 mg N2O m−2 h−1, and high N2O emissions occurred during spring (0.0278 mg N2O m−2 h−1) and winter (0.0139 mg N2O m−2 h−1) while low fluxes were observed during summer (0.0065 mg N2O m−2 h−1) and autumn (0.0060 mg N2O m−2 h−1). The annual average N2O flux from the intertidal zone was 0.0117 mg N2O m−2 h−1, and the cumulative N2O emission throughout a year was 113.03 mg N2O m−2, indicating that coastal marsh acted as N2O source. Over all seasons, N2O fluxes from the four marshes were significantly different (p < 0.05), in the order of HM (0.0256 ± 0.0040 mg N2O m−2 h−1) > MF (0.0107 ± 0.0027 mg N2O m−2 h−1) > LM (0.0073 ± 0.0020 mg N2O m−2 h−1) > MM (0.0026 ± 0.0011 mg N2O m−2 h−1). Temporal variations of N2O emissions were related to the vegetations (Suaeda salsa, Phragmites australis, and Tamarix chinensis) and the limited C and mineral N in soils during summer and autumn and the frequent freeze/thaw cycles in soils during spring and winter, while spatial variations were mainly affected by tidal fluctuation and plant composition at spatial scale. This study indicated the importance of seasonal N2O contributions (particularly during non-growing season) to the estimation of local N2O inventory, and highlighted both the large spatial variation of N2O fluxes across the coastal marsh (CV = 158.31 %) and the potential effect of exogenous nitrogen loading to the Yellow River estuary on N2O emission should be considered before the annual or local N2O inventory was evaluated accurately.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nitrous oxide (N2O) is an important greenhouse gas (GHG) that has 298 times the global warming potential of CO2 over a 100-year time period and has been recognized to contribute global warming by 5 % (Mosier 1998). N2O has an atmospheric lifetime of 114 years (Mosier 1998) and also contribute to the depletion of ozone in the stratosphere (Crutzen and Ehhalt 1977). The globally averaged atmospheric N2O concentration increased from 270 ± 7 ppb in pre-industrial times (before 1750) to 319 ± 0.12 ppb in 2005, and is increasing approximately 0.26 % per year (IPCC 2007). In 2010, the globally averaged N2O concentration reached 323.2 ppb, which exceeded the highest annual mean abundance so far (World Meteorological Organization 2011). Emission of N2O from various natural ecosystems has significant influences on global climate change since they account for 44~54 % of the total N2O emissions (9.6~10.8 Tg N2O year−1) (IPCC 2007). Tropical soil and wetlands play an important role in the global nitrogen (N) biogeochemical cycles and are considered significant natural sources of N2O, contributing approximately 22~27 % towards this inventory (Whalen 2005).
Coastal marsh is characterized by high temporal and spatial variation involved with topographic feature, environmental factors, and astronomic tidal fluctuation, and is very sensitive to global climate changes and human activities. The intertidal zone between terrestrial and coastal ecosystems may represent a high dynamic interface of intense material processing and transport, with potentially high GHG emission (Hirota et al. 2007). Considerable efforts have been made in the past two decades to quantify the N2O fluxes in different coastal ecosystems, especially in estuarine salt marshes (Shingo et al. 2000; Magalhães et al. 2007), mangrove swamps (Muñoz-Hincapié et al. 2002; Allen et al. 2007; Ganguly et al. 2008), coastal lagoons (Gregorich et al. 2006; Hirota et al. 2007), and coastal marshes (Amouroux et al. 2002; Moseman-Valtierra et al. 2011). In China, the studies on N2O emission from coastal marshes started quite late and the related research mainly focused on the coastal tundra marshes in Antarctica (Sun et al. 2002; Zhu et al. 2008) and the coastal marshes in the Yangtze River estuary (Yang et al. 2006; Wang et al. 2006, 2007a,b; Wang et al. 2010a) and the Min River estuary (Yang et al. 2012; Mou et al. 2012; Zhang et al. 2012, 2013), while information on the coastal marshes in northern estuaries (such as Liao River estuary and Yellow River estuary) was scarce.
The Yellow River is well-known as a sediment-laden river. Every year, approximately 1.05 × 107 tons of sediment is carried to the estuary and deposited in the slow flowing landform, resulting in vast floodplain and special marsh landscape (Xu et al. 2002; Cui et al. 2009). Sediment deposition is an important process for the formation and development of coastal marshes in the Yellow River Delta. The deposition rate of sediment in the Yellow River not only affects the formation rate of coastal marsh, but also, to some extent, influences the water or salinity gradient and the succession of vegetation from the land to the sea. In recent years, the N and organic matter (OM) loadings of the Yellow River estuary have significantly increased due to the effects of human activities, and approximately 4.22× 104 tons of nutrients and 4.39× 105 tons of OM were discharged into Bohai Sea every year (State Oceanic Administration of China 2013). Increases in N and OM loadings to estuarine and coastal marshes can stimulate microbial processes and associated GHGs emission (Seitzinger and Kroeze 1998; Purvaja and Ramesh 2001). However, information on N2O emission from the coastal marsh in the Yellow River estuary is very limited, and the potential effects of exogenous N loading on N2O emission remains poorly discussed.
In this paper, we investigated N2O fluxes and environmental variables in the coastal mash of the Yellow River estuary throughout a year (during 2010/2011). The aims of this study are: (1) to determine the spatial and temporal variations of N2O fluxes and the annual average N2O emission from the coastal marsh, and (2) to investigate the key factors influencing N2O variations and assess the potential effects of exogenous N loading on N2O emission.
Materials and methods
Study site
The study was carried out in the coastal marsh of the Yellow River estuary, which is located in the Nature Reserve of Yellow River Delta (37°35′N~38°12′N, 118°33′E~119°20′E) in Dongying City, Shandong Province, China. The nature reserve is of typical continental monsoon climate with distinctive seasons. The annual average temperature is 12.1 °C, and the average temperatures in spring, summer, autumn, and winter are 10.7, 27.3, 13.1, and −5.2 °C, respectively. The temperature changes significantly during early spring and winter, and the freeze/thaw cycles frequently occur in topsoil in majority days, with the frozen depth ranged from 0 to 15 cm. The annual evaporation is 1,962 mm, the annual precipitation is 551.6 mm, with about 70 % of precipitation occurring between June and August.
With an area of 964.8 km2, coastal marsh is the main type of marsh in the Yellow River Delta and accounts for 63.06 % of total area (Cui et al. 2009). In the intertidal zone, natural geomorphology and depositing zones are distinct, and high marsh (HM), middle marsh (MM), low marsh (LM), and mudflat (MF) develop from the land to the sea. The soil in the intertidal zone is dominated by salt soil. Suaeda salsa, an annual C3 plant, is the most prevalent halophytes in the coastal marshes of the Yellow River estuary (Tian et al. 2005). Due to the differences of water and salinity conditions, three S. salsa phenotypes are generally formed in HM, MM, and LM, respectively. The HM is predominated by S. salsa (>90%) and Phragmites australis (<10%), MM is predominated by S. salsa (>95%) and Tamarix chinensis (<5%), while LM is pure S. salsa community (100%) (Mou 2010). The tide in the intertidal zone of the Yellow River estuary is irregular semidiurnal tide (twice a day) and the mean tidal range is 0.73~1.77 m (Li et al. 1991).
Experimental design
Four sampling positrons were laid in HM, MM, LM, and MF in the intertidal zone of the Yellow River estuary. N2O fluxes across the sediment–atmosphere interface were measured by using opaque, static, manual stainless steel chambers, and gas chromatography techniques. The chamber (50 × 50 × 50 cm) and its base (50 × 50 × 20 cm) were made from 0.4-mm thickness stainless steel. Inside the chamber, an electric fan was fixed to stir the air, a thermometer sensor was installed to measure temperature, a trinal-venthole was fixed to collect gas sample, and a balance pipe was used to equalize the air pressure between the inside and the outside of the chamber. Outside of the chamber was covered with 2 cm thickness white foam to reduce the impact of direct radiative heating during sampling, which generally caused very little change in temperature between the inside and the outside of the chamber (Teiter and Mander 2005; SØvik and KlØve 2007; Song et al. 2008; Jiang et al. 2010). All the connections were made “air tight” and sealed by silicon rubber. The stainless steel bases enclosed an area of 0.25 m2 and were inserted into the ground to a depth of 20 cm below the soil on August 2010. During observations, the chamber was placed over the base filled with water in the groove to prevent leakage, and the plant was covered within the chamber.
Sampling campaigns were undertaken in September, October, November, and December in 2010, and April, May, June, and July in 2011 (the sampling in January, February, and March were canceled due to the frequent effects of storm tide and bad weather and that in August was canceled due to the damage of most chambers and instruments). Each measurement campaign consisted of 12 chambers set up at above-mentioned four positions (three chambers per site). On each sampling date, measurements were conducted at 0700, 0930, 1200, 1430, and 1700 hours (represented different times of day). About 60 ml gas sample inside the chamber was collected every 20 min over a 60-min period by using 100-ml syringe (total of four samples), and then stored in pre-evacuated gas sampling bags (100 ml). Since the tide in the Yellow River estuary is irregular semidiurnal tide, the sampling campaigns in the LM and MF were sometimes affected by tidal inundation. The sampling campaigns in the LM and MF in May and the MF in June were not carried out due to the great influence of tide.
N2O concentrations of gas samples were analyzed within 36 h using gas chromatography (Agilent 7890A) equipped with ECD. The N2O portion was separated using a 1-m stainless steel column with an inner diameter 2-mm Porapak Q (80/100 mesh), and was measured using the ECD, which was set at 330 °C. The ECD also used high-pure nitrogen as a carrier gas, a flow rate of 35 ml min−1. The column temperatures were maintained at 55 °C for all separations. Gas concentrations were quantified by comparing peak areas of samples against standards run every eight samples, ensuring each sample run maintained RSD below 6 %.
N2O fluxes were calculated according to the following equation:
where dc/dt is the slope of the gas concentration curve variation along with time. M is the mole mass of each gas. P is the atmospheric pressure in the sampling site. T is the absolute temperature during sampling. V 0, T 0, P 0 are the gas mole volume, air absolute temperature, and atmospheric pressure under standard conditions, respectively. H is the height of chamber above the ground/water surface.
The annual average N2O flux was calculated by the data determined in the eight sampling months (covered four seasons). The N2O emission/absorption (milligram of N2O per meter squared) per sampling month was calculated by the average value (milligram of N2O per meter squared per hour) of all sampling data and the time (hour) in each month. The N2O emissions/absorptions in January and February were estimated by the average value in winter (December), and those in March and August were estimated by the average values determined in spring (April and May) and summer (June and July), respectively. The cumulative N2O emission/absorption throughout a year was estimated by the values in 12 months.
Environmental measurements
Air temperature and soil temperatures (0, 5, 10, and 15 cm) were measured in each position during gas sampling. Soil volumetric moisture and electrical conductivity (EC) in 0–5 and 5–10 cm depths were determined in situ by high-precision moisture measuring instrument (AZS-2) and soil and solution EC meter (Field Scout), respectively. Soil moisture and EC were not determined in December 2010 since the topsoil was frozen. Because the measurements in LM and MF in September 2010 were partly and slightly affected by tide (from 1200 to 1700 hours), the flooding depths were measured to discuss the effects of tidal inundation on N2O emission. On each sampling date, two soil samples per layer (0–10, 10–20 cm) were taken in each site for analyzing TC and TN contents by element analyzer (Elementar Vario Micro, German) and NH4 +–N and NO3 −–N contents by sequence flow analyzer (San++ SKALAR, Netherlands).
Statistical analysis
The Shapiro–Wilk test was applied to identify the normality of data before the related statistical analyses were conducted. The results were presented as means of the replications, with standard error. Statistical significance of differences at p < 0.05 between samples was analyzed using analysis of variance (ANOVA). Multiple comparison of samples was undertaken by Tukey’s test with a significance level of p = 0.05. Correlation analyses and stepwise linear regression analyses were used to examine the relationship between fluxes and the measured environmental variables. In all tests, differences were considered significantly only if p < 0.05.
Results
Environmental variables in coastal marsh
Similar variations of air temperature and ground temperature in the four marshes were observed over all sampling period (Fig.1a). Air temperature did not show significant difference among the four marshes (p > 0.05) and the means were 19.69, 17.90, 15.74, and 15.00 °C, respectively. Ground temperatures generally decreased with increasing soil depth, but no significant differences were found within the four marshes (p > 0.05). Dissimilar variations of soil moisture and EC in the four marshes were observed (Fig.1b, c). With increasing depth, soil moisture increased while EC generally decreased. Soil moisture did not show significant differences among the four marshes (p > 0.05), while significant differences of EC were observed (p < 0.05). Seasonal dynamics of soil substrate in the four marshes were observed over all sampling period (Fig. 2). TC, TN, and NH4 +–N in the surface and subsurface soil of MF were generally higher than those in other marshes (Fig.2a–c). Both TC in surface and subsurface soil had significant differences within the four marshes (p < 0.05), while only TN in subsurface soil showed significant difference (p < 0.01). Both NH4 +–N and NO3 −–N in soil were not significantly different among the four marshes (p > 0.05).
Spatial variations of N2O fluxes
Variation of N2O fluxes in spring
N2O fluxes in spring averaged between −0.0102 and 0.0982 mg N2O m−2 h−1 and differed significantly among the four marshes (p < 0.01) (Fig. 3). With the exception of MM, the other sites were found to release N2O during all times of day sampled. In HM, N2O fluxes in April and May were similar except for 1700 hours sampling, and significantly higher emission occurred in April compared to May (p < 0.01). N2O fluxes from MM in April and May were opposite except for 1200 hours sampling, and the ranges were −0.0065~0.0131 and −0.0102~0.0081 mg N2O m−2 h−1, respectively. N2O fluxes from LM in April had no significant variation before 1430 hours and a significant peak occurred in 1700 hours. In MF, N2O fluxes in April ranged from 0.0285 to 0.0512 mg N2O m−2 h−1 and the maximum occurred in 1200 hours. The mean N2O fluxes from HM, MM, LM, and MF in spring were 0.0582, 0.0026, 0.0069, and 0.0384 mg N2O m−2 h−1, and the cumulative N2O emissions were 128.02, 5.75, 15.19, and 84.74 mg N2O m−2, respectively, indicating that coastal marsh represented N2O source.
Variation of N2O fluxes in summer
N2O fluxes in summer ranged from −0.0147 to 0.0262 mg N2O m−2 h−1 and differed significantly among the four marshes in June (p < 0.05) (Fig. 3). With the exception of HM that were found to release N2O during all times of day sampled, the other sites showed consumptions in some sampling times. N2O fluxes from HM in June and July were 0.0085~0.0262 and 0.0117~0.0206 mg N2O m−2 h−1, respectively, and no significant difference was found between them (p > 0.05). In MM, N2O fluxes in June and July were significantly different (p < 0.05) and the means were −0.0051 and 0.0052 mg N2O m−2 h−1, respectively. Although N2O fluxes from LM in June and July were opposite except for 1430 hours sampling, they had no significant difference (p > 0.05). The MF was found to release N2O before July 1430 hours sampling and significant consumption occurred in 1700 hours. The average N2O fluxes from HM, MM, LM, and MF in summer were 0.0165, 0.00002, 0.0035, and 0.0055 mg N2O m−2 h−1, and the cumulative N2O emissions were 36.54, 0.17, 7.92, and 12.14 mg N2O m−2, respectively, indicating that coastal marsh acted as weak N2O source.
Variation of N2O fluxes in autumn
N2O fluxes in autumn averaged between −0.0092 and 0.0312 mg N2O m−2 h−1 and differed significantly among the four marshes in October (p < 0.01) (Fig. 3). In HM, with the exception of September 0700 hours sampling, the other times were found to release N2O. Significantly higher emission occurred in October compared to September and November (p < 0.01). N2O fluxes from MM in autumn showed both emission and consumption, but no significant difference was found among sampling periods (p > 0.05). N2O fluxes from LM in September and October were opposite except for 0700 and 0930 hours sampling. N2O fluxes from MF in September and November were similar, but they had significant difference (p < 0.01). The LM in November and MF in October were found to release N2O over all sampling times, with the maximums occurred in 1200 and 0700 hours, respectively. The mean N2O fluxes from HM, MM, LM, and MF in autumn were 0.0118, 0.0031, 0.0038, and 0.0053 mg N2O m−2 h−1, and the cumulative N2O emissions were 26.01, 6.90, 8.16, and 11.70 mg N2O m−2, respectively, indicating that coastal marsh represented weak N2O source.
Variation of N2O fluxes in winter
N2O fluxes in winter ranged from −0.0114 to 0.0442 mg N2O m−2 h−1and differed significantly among the four marshes (p < 0.01) (Fig. 3). The average N2O fluxes from HM, MM, LM, and MF were 0.0195, 0.0059, 0.0257, and 0.0047 mg N2O m−2 h−1, and the cumulative N2O emissions were 42.12, 12.79, 55.47, and 10.11 mg N2O m−2, respectively, indicating that coastal marsh acted as N2O source. Significantly higher emissions were observed in HM and LM compared to MM and MF during all times of day sampled (p < 0.05).
Temporal variations of N2O fluxes
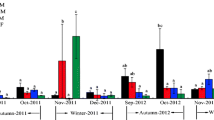
Significant temporal variations of N2O fluxes throughout a year were observed in HM, LM, and MF (p < 0.01) (Fig. 4). During all times of day and the seasons measured, N2O fluxes from the intertidal zone averaged between −0.0051 and 0.0805 mg N2O m−2 h−1, and the annual average N2O flux was 0.0117 mg N2O m−2 h−1. The maximum and minimum were observed in April (in HM) and June (in MM), respectively. High N2O emissions generally occurred during spring (0.0278 mg N2O m−2 h−1) and winter (0.0139 mg N2O m−2 h−1) while low fluxes were observed during summer (0.0065 mg N2O m−2 h−1) and autumn (0.0060 mg N2O m−2 h−1) (Fig. 4). Over all seasons, N2O fluxes from the four marshes were significantly different (p < 0.05), in the order of HM (0.0256 ± 0.0040 mg N2O m−2 h−1) > MF (0.0107 ± 0.0027 mg N2O m−2 h−1) > LM (0.0073 ± 0.0020 mg N2O m−2 h−1) > MM (0.0026 ± 0.0011 mg N2O m−2 h−1). The cumulative N2O emission from the intertidal zone throughout a year was 113.03 mg N2O m−2, indicating that coastal marsh acted as N2O source. Comparatively, the N2O emissions measured during spring and winter contributed 49.65 and 26.65 % of the total emission, respectively, which were higher than the contributions measured during summer (12.03 %) and autumn (11.67 %).
Temporal variations of N2O fluxes (milligram of N2O per meter squared per hour) from high marsh (HM), middle marsh (MM), low marsh (LM), and mudflat (MF). Bars with different letters (a, b, c, d for HM; x, y for MM; m, n for LM; o, p, q for MF) are significantly different at the level of p < 0.05; bars with same letters are not significantly different at the level of p < 0.05
Relationships between environmental variables and N2O fluxes
Most correlations between N2O fluxes and temperatures in different marshes were not significant (p > 0.05; Table 1). Although both positive and negative impacts of soil moisture (or EC) on N2O emissions were observed within the four marshes, only the correlation between soil moisture (5–10 cm) and N2O fluxes in LM was significant (p < 0.05; Table 2). Lacks of correlations between N2O fluxes and substrate variables were observed (p > 0.05) except the correlations occurred in subsurface soil of HM and MF (p < 0.05; Table 3). The environmental variables determined in the four coastal marshes were all excluded in the stepwise liner regression. NH4 +–N content (X 1) and TC content (X 2) were the dominant factors that controlled the N2O emissions (Y) in HM (Y = 0.129 − 0.040X 1, R 2 = 0.539, p = 0.038) and LM (Y = −0.109 + 0.090X 2, R 2 = 0.798, p = 0.016), respectively, while in MM and MF, the environmental variables determined during sampling periods were all excluded, indicating that N2O fluxes were controlled by multiple site-specific factors.
Discussion
Temporal variations of N2O fluxes
Many studies have demonstrated that the seasonal patterns of N2O fluxes were generally governed by seasonal variability in temperatures since it influenced water availability, production of substrate precursors, and microbial activity (Whalen 2005; Zhu et al. 2008). However, we have drawn a different result. Although significant temporal variations in N2O fluxes from the coastal marshes in the Yellow River estuary were observed, the influence of seasonal variability in temperatures on N2O emissions seemed not significant (Figs. 1a and 4). Moreover, most correlations between N2O fluxes and temperatures in different marshes were not significant (p > 0.05) (Table 1). These indicated that the influences of temperatures on N2O emissions might be covered by other biotic/abiotic parameters (such as vegetation, soil moisture, and substrate) in most sampling periods. Because the environmental variables determined in coastal marsh were all excluded in the stepwise liner regression, we considered that N2O emissions in different seasons might be controlled by the interactions of multiple controlling factors.
In this paper, we observed that N2O emissions in spring, summer, autumn, and winter were 0.0278, 0.0065, 0.0060, and 0.0139 mg N2O m−2 h−1, respectively (Fig. 4), and low values occurred during summer and autumn. The result was similar with Allen et al. (2007) but was different with Wang et al. (2007a) and Søvik and Kløve (2007). There were two possible reasons. Firstly, the presence of vegetations (P. australis, S. salsa, and T. chinensis) across the coastal marsh might have significant effects on the low N2O emissions during the growing season. Many studies have demonstrated that, under flooding or anaerobic conditions, the three mash plants could transport oxygen from aboveground parts to roots by aerenchyma (Han et al. 2005; Ling et al. 2008; Kong et al. 2008; Ge and Zhang 2011), which generally formed oxidizing microenvironment around rhizosphere soil (Kong et al. 2008). Moreover, the roots of the three plants could excrete some small molecular compounds (glucide, organic acid, and amion acid), which caused the microorganism amount and microbial activity in rhizosphere soil to be much higher than those in non-rhizosphere soil (Ling et al. 2008; Cheng 2009; Wang et al. 2010b; Ge and Zhang 2011). These indicated that although the coastal marshes were frequently influenced by tidal inundation, the nitrification–denitrification rate still could be accelerated by the three plants since the soil microbes in rhizosphere were generally supplied with available C and N and proper amount of oxygen. Thus, N2O, to a great extent, was reduced to N2 by denitrification regardless of whether N2O was produced by nitrification or denitrification or both, which resulted in the great decrease of N2O emissions. Secondly, as mentioned before, N2O production was through nitrification and denitrification, in which microorganism used the C and mineral N (NH4 +–N and NO3 −–N) as substrates (Conrad 1996). The low N2O emissions in summer might also be due to the limited C and mineral N in the soils caused by low mineralization rates of organic C and N. As shown in Fig. 2, compared to other seasons, TC and TN in different coastal marsh soils (0–10, 10–20 cm) were lower in summer and NH4 +–N were lower during spring and summer, indicating that the shortage of C and mineral N during summer was unfavorable for N2O production. Chapuis-lardy et al. (2007) also pointed out that N2O production seemed to be suppressed by low mineral N and high moisture contents in soil from analysis a large number of literatures. The weak N2O emissions in summer were probably because that the mineral N was almost used up by plants. This speculation could be supported by the evidence that the biggest biomass accumulation rate (Mou et al. 2010) coincided with the lowest level of C and mineral N in the soils (Fig. 2) at this period. This study also showed that high N2O emissions occurred during spring and winter (Fig. 4). Many studies have demonstrated that, in the mid-high latitude and high altitude regions, freeze/thaw cycle occurred in late autumn, winter, or early spring was a very important process to increase N2O production and emission since it could affect soil physical and biological properties greatly (Teepe et al. 2001; Song et al. 2008). Because the coastal marsh of the Yellow River estuary located in the mid-latitude region (37 °35′N~38 °12′N) and the freeze/thaw cycles frequently occurred in topsoil in majority days during spring and winter (frozen depth, 0~15 cm), high N2O emissions might be attributed to the frequent freeze/thaw cycles. For one thing, frequent freeze/thaw cycles destroyed the size and stability of soil aggregate (van Bochove et al. 2000), released abundant bioavailable C and N (Ludwig et al. 2006), and altered the course and intensity of soil N transformation (Jarvis et al. 1996), which enhanced the denitrification and N2O emission (Priemé and Christensen 2001). For another, since the frozen water film on the soil matrix represented a diffusion barrier which reduced oxygen supply to the microorganisms and partly prevented the release of the N2O (Teepe et al. 2001), high emissions occurred due to the quick release of N2O trapped by ice layer and/or denitrification during frequent freeze/thaw cycles (Goodroad and Keeney 1984; Teepe et al. 2001). Similar results were drawn by Zhang et al. (2005) and Jiang et al. (2010) who found significant N2O emissions from freshwater marsh in the Sanjiang Plain and Alpine meadow in the Qinghai–Tibetin Plateau during the freeze/thaw cycle as the temperature increased.
In this study, the N2O emission per sampling month was calculated by the average value and the time in each month and the emissions in absent months were estimated by the average values in the corresponding seasons. Similar method was adopted in some studies to estimate the N2O emissions in absent months. Hao et al. (2007) studied the effects of freshwater marshes (Carex lasiocapa marsh and Deyeuxia angustifolia marsh) reclamation on N2O fluxes and estimated the emission in absent month (February) by the average value in winter (December and January). Sun et al. (2009) investigated the N2O fluxes from Calamagrostis angustifolia marsh in the Sanjiang Plain and estimated the emissions in absent months (February and October) by the average values in winter (December and January) and autumn (September and November), respectively. Since the measurements in this study covered four seasons [spring (April and May), summer (June and July), autumn (September, October, and November), and winter (December)], we considered that based on the average values in the corresponding seasons, the extrapolation of N2O emissions in absent months, to a great extent, was valid as the local N2O inventory throughout a year was assessed roughly. Although only the roughly accumulated N2O emission was given in our study, considering it was estimated for the first time, it still would provide valuable information on understanding the N2O inventory in the coastal marsh of the Yellow River estuary. Overall, across all the seasons sampled, the coastal marsh was a net source of N2O (113.03 mg N2O m−2 year−1) and the N2O emissions measured during non-growing season (spring and winter) comprised the principal part to the total emission (76.30 %), indicated that the importance of seasonal N2O contributions (particularly during non-growing season) to the estimation of local N2O inventory should be paid more attentions. In the following studies, in order to assess the regional budget of N2O emissions precisely, measurements should be conducted at all months and the sampling frequency should be increased.
Spatial variations of N2O fluxes
During all times of day and the seasons measured, we found that the physical and chemical parameters of soil differed in their magnitude among the four marshes, and significant differences in TC, TN, and EC in soil were observed (p < 0.05). Such differences within the four marshes would be due to the site-specific conditions (such as topography, slope, hydrology, and species composition) which influence the magnitudes and variations of N2O at spatial scale (Allen et al. 2007; Hirota et al. 2007). Over all sampling seasons, we observed that N2O fluxes from the four marshes were significantly different (p < 0.05), in the order of HM > MF > LM > MM (Fig. 4). Also, a large spatial variation of N2O fluxes in the coastal marsh of the Yellow River estuary was observed. The coefficient of variations (CVs) of N2O fluxes in the four marshes were 98.47, 278.29, 164.56, and 138.12 %, respectively, while the value across the coastal marsh was 158.31 %, indicating that to assess the regional budget of N2O emissions precisely, measurements should be designed at fine scales and the number of spatial replicates should be increased. Previous studies have indicated that temperatures had great influences on N2O emissions at spatial scale (Alongi et al. 2005; Gregorich et al. 2006), but this study has drawn a different result. During all the seasons measured, air temperature and ground temperatures did not show significant difference among the four marshes (p > 0.05), most correlations between N2O fluxes and temperatures in different marshes were not significant (p > 0.05) and only few significant positive or negative correlations were found in HM or MM (Table 1). This indicated that the function of thermal condition might be covered by the interactions of other biotic or abiotic factors (such as moisture, salinity, sediment substrate, and vegetation) though it was considered an important factor affecting N2O emission. Although EC showed significant differences within the four marshes (p < 0.05), the correlations between EC and N2O emission were not significant (p > 0.05) (Table 2). By comparison, soil moisture did not show significant differences among the four marshes (p > 0.05), but significant positive correlation between soil moisture (5–10 cm) and N2O emission was found in LM (p < 0.05) (Table 2). Generally, both positive and negative impacts of soil moisture (or EC) on N2O emissions were observed in coastal marshes (Table 2), which were different with mostly previous studies that N2O emissions had negative correlation with moisture (Regina et al. 1996; Wang et al. 2005) or EC (Law et al. 1991; Dalal et al. 2003; Chen et al. 2010). One possible explanation for the positive correlations between EC and N2O emissions was that the salinity in LM and MF was much lower than that in HM and MM (Fig.1c), which might not completely inhibit the activities of nitrifiers and denitrifiers in soil (Lv et al. 2008). The positive correlations between soil moisture and N2O emissions might be partly dependent on the fluctuation of soil moisture (or water level) by astronomic tide. As shown in Fig. 5, dissimilar variations of N2O emissions and flooding depths in LM and MF were observed. Both LM and MF were found to release N2O at 0700 and 0930 hours sampling (before flood), indicating that the proper soil moisture might contribute to a favorable aerobic–anerobic status for N2O production. As the flood began at 1200 hours sampling, the N2O flux in LM decreased and the value in MF became negative, indicating that the soil moisture in LM and MF might be greatly changed due to the different flooding depths, which produced different impacts on N2O emission. When the flooding was deeper at 1430 hours sampling, both LM and MF showed great consumptions, indicating that the tidal inundation produced an unfavorable anaerobic status for N2O production and the limited N2O emission might be severely prevented by flooding seawater. Similar result was drawn by Zhang et al. (2013) who found that tidal inundation significantly decreased the N2O emission in the coastal marsh of the Min River estuary. Although the flooding depth in LM decreased and that in MF increased greatly at 1700 hours sampling, both LM and MF were found to release N2O greatly, which was mainly dependent on the N2O transportation from surface seawater to the two marshes by tidal fluctuation (Senga et al. 2001; Hirota et al. 2007). In addition, the decrease of flooding depth in LM might cause the dissolved N2O in seawater to be released, which partly contributed to the significant N2O emission. Similar result was drawn by Hirota et al. (2007) who found that in coastal ecosystems subjected to such short-term fluctuation of water level (or soil moisture) by astronomic tide, the spatial variations in N2O flux was controlled by fluctuation of water lever (r = 0.58, p < 0.05).
Site-level control of N2O emission was also attributed to the effects of vegetation and nutrient status. In this study, the plant distributed continuously across the coastal marsh and the plant compositions in the four mashes were different. The coverage and maximum biomass of S. salsa–P. australis community (HM) were 1.19- and 1.90-folds and 1.60- and 3.57-folds of S. salsa–T. chinensis community(MM) and S. salsa community (LM), respectively (Mou 2010; Dong et al. 2010). Also, the presence of P. australis, T. chinensis, and S. salsa had great impacts on N2O emission as mentioned previously. These indicated that the vegetations across the coastal marsh might play an important role in controlling the N2O emissions at spatial scale. Although both TC and TN in soils showed significant differences among the four marshes (p < 0.05), lacks of correlations between N2O fluxes and TC (or TN) were observed (p > 0.05) (Table 3). By comparison, although both NH4 +–N and NO3 −–N had no significant differences within the four marshes (p > 0.05), significant correlations between N2O fluxes and NH4 +–N could be observed in subsurface soil of HM and MF (p < 0.05) (Table 3). C and N were very important substrates for N2O production (participated in nitrification and denitrification processes) (Tauchnitz et al. 2008) and they generally influenced N2O emissions by C/N regulations and interactions with other abiotic variables (Blackwell et al. 2010). In this study, negative correlations between N2O emissions and nutrient variables were generally observed in the four marshes (Table 3), which was different with mostly previous studies (Aelion et al. 1997; Muñoz-Hincapié et al. 2002; Chen et al. 2010). One possible reason was related to the interaction of vegetation and microorganism (nitrifiers and denitrifiers) during N2O production (Li et al. 2002). N2O production might be greatly inhibited as the available N was significantly competed by both vegetations and microorganisms in S. salsa–P. australis community, S. salsa–T. chinensis community, and S. salsa community, which partly contributed to the difference of N2O emissions at spatial scale. Since there was no vegetation in MF, the negative correlations between N2O emissions and nutrient variables were possibly correlated with the high soil moisture (Fig.1b). Under high moisture condition, the NO3 −–N in topsoil could be easily transferred to the deep soil layer, which decreased the chance to participate in nitrification and denitrification processes. As shown in Fig.2d, the NO3 −–N in topsoil of MF was very low, which could partly explain the negative influences on N2O production and emission.
Comparisons with other measurements and potential of N loading on N2O emissions
Previous studies that have examined the release of N2O from different coastal marshes and mangrove swamps reported about the fluxes in the range of −0.1298 to 0.1953 mg N2O m−2 h−1 (Alongi et al. 2005; Xie et al. 2011) (Table 4). The magnitudes of N2O fluxes determined in this study (−0.0051~0.0805 mg N2O m−2 h−1) were in the range, which were higher than those from coastal marshes in the Yangtze River estuary (−0.0096~0.0079 mg N2O m−2 h−1) and the Min River estuary (0.0037~0.0157 mg N2O m−2 h−1), and mangrove swamps in the Moreton Bay (−0.002~0.014 mg N2O m−2 h−1), but matched emissions recorded at coastal tundra marshes in the eastern Antarctica (−0.0206~0.0856 mg N2O m−2 h−1), coastal marshes in the coastal lagoon of Lake Nakaumi (−0.01~0.06 mg N2O m−2 h−1) and mangrove swamps in the Brisbane River (−0.004~0.065 mg N2O m−2 h−1) and Magueyes Island (0.0022~0.0616 mg N2O m−2 h−1) (Table 4).
In this paper, we found that the coastal marsh acted as a N2O source (cumulative N2O emission throughout a year was 113.03 mg N2O m−2) in the present N loading of the Yellow River estuary. Numerous studies have demonstrated that exogenous N generally had great stimulatory effects on the production and emission of N2O (Lee et al. 1997; Muñoz-Hincapié et al. 2002; Liikanen et al. 2003; Song et al. 2006; Zhang et al. 2007; Stadmark and Leonardson 2007; Li et al. 2009, 2010; Zhang et al. 2012, 2013), but the promoted magnitude of N2O flux to N enrichment varied due to the N addition level (Song et al. 2006; Zhang et al. 2007; Li et al. 2009, 2010; Zhang et al. 2012; Mou et al. 2012) and N forms (NH4 +–N and NO3 −–N) (Smith et al. 1983; Lindau and DeLaune 1991; Cartaxana and Lloyd 1999; Muñoz-Hincapié et al. 2002; Wan et al. 2009). Wang (2011) studied the responses of N enrichment (NH4 +–N) on the N2O production of coastal marsh soil in the Yellow River estuary, and found that the additions of NH4 +–N had great stimulation on N2O production, with approximately 1.93~3.71-folds of N2O production being observed with increasing NH4 +–N addition. Denitrification was the most important process for N2O production and its contribution to total N2O production would also be elevated with increasing NH4 +–N addition (Wang 2011). The increase in N2O emission under N addition was probably caused by the enhancement of both nitrifiers and denitrifiers activities (Wrage et al. 2004). At present, the exogenous N loading (NH4 +–N is dominated) of the Yellow River estuary is increasing due to human activities (State Oceanic Administration of China 2013). Since N is a very limited nutrient in the coastal marshes of the Yellow River estuary (Mou 2010), increases in exogenous N loading to estuarine and coastal marshes will stimulate microbial processes and N2O emission. As shown in Table 4, the mean N2O flux from Futian mangrove (0.7572 mg N2O m−2 h−1) and the maximum N2O emission from Mai Po mangrove (0.4176 mg N2O m−2 h−1) in the Deep Bay region of South China recorded by Chen et al. (2010) were 9.41 and 5.19 times greater than the maximum N2O emission reported by our study, and the reason was probably dependent on the higher nutrient loadings of the Deep Bay region compared to the Yellow River estuary. According to the Ocean Environmental Quality Communique of China in 2012, approximately 4.22 × 104 tons of nutrients and 4.39 × 105 tons of OM were discharged into Bohai Sea by Yellow River, while approximately 6.42 × 105 tons of nutrients and 4.65 × 105 tons of OM were imported into Deep Bay region by Pearl River (State Oceanic Administration of China 2013). Because the Futian and Mai Po mangroves were located in inner Deep Bay receiving discharges from the Pearl River Delta and nearby polluted rivers in Shenzhen and Hong Kong (Ong Che 1999), significantly higher fluxes of N2O were mainly related to the high nutrient inputs from the polluted rivers that flooded into Deep Bay, such as Pearl River (Chen et al. 2010).
Based on the above analysis, we concluded that the N2O emission in the future will be enhanced with increasing N loading to the Yellow River estuary (especially NH4 +–N is the major pollutant) and denitrification will play a very important role in contributing the total N2O emission. With increasing N loading, the magnitude of N2O emission in the Yellow River estuary should be paid more attention as the annual or local N2O inventory was assessed accurately.
References
Aelion CM, Shaw JN, Wahl M (1997) Impact of suburbanization on ground water quality and denitrification in coastal aquifer sediments. J Exp Mar Biol Ecol 213:31–51
Allen DE, Dalal RC, Rennenberg H, Meyer RL, Reeves S, Schmidt S (2007) Spatial and temporal variation of nitrous oxide and methane flux between subtropical mangrove sediments and the atmosphere. Soil Biol Biochem 39:622–631
Alongi DM, Pfitzner J, Trott LA, Tirendi F, Dixon P, Klumpp DW (2005) Rapid sediment accumulation and microbial mineralization in forests of the mangrove Kandelia candel in the Jiulongjiang Estuary, China. Estuar Coast Shelf S 63:605–618
Amouroux D, Roberts G, Rapsomanikis S, Andreae MO (2002) Biogenic gas (CH4, N2O, DMS) emission to the atmosphere from near-shore and shelf waters of the North-western Black Sea. Estuar Coast Shelf S 54(3):575–587
Bauza JF, Morell JM, Corredor JE (2002) Biogeochemistry of nitrous oxide production in the red mangrove (Rhizophora mangle) forest sediments. Estuar Coast Shelf S 55:697–704
Blackwell MSA, Yamulki S, Bol R (2010) Nitrous oxide production and denitrification rates in estuarine intertidal saltmarsh and managed realignment zones. Estuar Coast Shelf S 87:591–600
Cartaxana P, Lloyd D (1999) N2, N2O and O2 profiles in a tagus estuary salt marsh. Estuar Coast Shelf S 48(6):751–756
Chapuis-Lardy L, Wrage N, Metay A, Chotte JL, Bernoux M (2007) Soils, a sink for N2O? A review. Global Change Biol 13:1–177
Chen GC, Tam NFY, Ye Y (2010) Summer fluxes of atmospheric greenhouse gases N2O, CH4 and CO2 from mangrove soil in South China. Sci Total Environ 408:2761–2767
Cheng WX (2009) Rhizosphere priming effect: its functional relationships with microbial turnover, evapotranspiration, and C-N budgets. Soil Biol Biochem 41:1795–1801
Conrad R (1996) Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO). Microbiol Rev 60:609–640
Crutzen PJ, Ehhalt DH (1977) Effects of nitrogen fertilizers and combustion on the stratospheric ozone layer. Ambio 6:112–117
Cui BS, Yang QC, Yang ZF, Zhang KJ (2009) Evaluating the ecological performance of wetland restoration in the Yellow River Delta, China. Ecol Eng 35:1090–1103
Dalal RC, Wang WJ, Robertson GP, Parton WJ (2003) Nitrous oxide emission from Australian agricultural lands and mitigation options: a review. Aust J Soil Res 41:165–195
Dong HF, Yu JB, Sun ZG, Mou XJ, Chen XB, Mao PL, Wu CF, Guan B (2010) Spatial distribution characteristics of organic carbon in the soil-plant systems in the Yellow River estuary tidal flat wetland. Environ Sci 31(6):1594–1599
Ganguly D, Dey M, Mandal SK, De TK, Jana TK (2008) Energy dynamics and its implication to biosphere–atmosphere exchange of CO2, H2O and CH4 in a tropical mangrove forest canopy. Atmos Environ 42:4172–4184
Ge LP, Zhang H (2011) Characteristics of soil enzymatic activities and relationship with the main nutrient in the rhizosphere of four littoral halophytes. Ecol Environ Sci 20(2):270–275
Goodroad LL, Keeney DR (1984) Nitrous oxide emissions from soils during thawing. Can J Soil Sci 64:187–194
Gregorich EG, Hopkins DW, Elberling B, Sparrow AD, Novis P, Greefield LG, Rochette P (2006) Emission of CO2, CH4 and N2O from lakeshore soils in an Antarctic dry valley. Soil Biol Biochem 38:3120–3129
Han N, Shao Q, Lu CM, Wang BS (2005) The leaf tonoplast V-H+-ATPase activity of a C3 halophyte Suaeda salsa is enhanced by salt stress in a Ca-dependent mode. J Plant Physiol 162:267–274
Hao QJ, Wang YS, Song CC, Jiang CS (2007) Effects of marsh reclamation on methane and nitrous oxide emissions. Acta Ecol Sin 27(8):3417–3426
Hirota M, Senga Y, Seike Y, Nohara S, Kunii H (2007) Fluxes of carbon dioxide, methane and nitrous oxide in two contrastive fringing zones of coastal lagoon, Lake Nakaumi, Japan. Chemosphere 68:597–603
IPCC (2007) Changes in atmospheric constituents and in radioactive forcing. In: Climate change: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge
Jarvis SC, Stockdale EA, Shepherd MA, Powlson DS (1996) Nitrogen mineralization in temperate agricultural soils: processes and measurement. Adv Agron 57:187–235
Jiang CM, Yu GY, Fang HJ, Cao GM, Li YN (2010) Short-term effect of increasing nitrogen deposition on CO2, CH4 and N2O fluxes in an alpine meadow on the Qinghai-Tibetan Plateau, China. Atmos Environ 44:2920–2926
Kong Y, Wang Z, Gu YJ, Wang YX (2008) Research progress on aerenchyma formation in plant roots. Chin Bull Bot 25(2):248–253
Kreuzwieser J, Buchholz J, Rennenberg H (2003) Emission of methane and nitrous oxide by Australian mangrove ecosystems. Plant Biol 5:423–431
Law CS, Rees AP, Owens NJP (1991) Temporal variability of denitrification in estuarine sediments. Estuar Coast Shelf S 33:37–56
Lee RY, Joye SB, Roberts BJ, Valiela I (1997) Release of N2 and N2O from salt-marsh sediments subject to different land-derived nitrogen loads. Biol Bull 193(2):292–293
Li J, Yu Q, Tong XJ (2002) Vegetation—an important source of atmospheric N2O. Earth Sci Front 9(1):112
Li YC, Song CC, Hou CC, Song YY (2010) Dynamics of N2O emission and carbon mineralization to exogenous nitrogen input in meadow marsh soil. J Soil Water Conserv 24(4):140–147
Li YC, Song CC, Liu DY, Wang L (2009) Denitrification loss and N2O emissions from different nitrogen inputs in meadow marsh soil. Res Environ Sci 22(9):1103–1107
Li YF, Huang YL, Li SK (1991) A primarily analysis on the coastal physiognomy and deposition of the modern Yellow River Delta. Acta Oceanol Sin 13(5):662–671
Liikanen A, Ratilainen E, Saarnio S, Alm J, Martikainen PJ, Silvola J (2003) Greenhouse gas dynamics in boreal, littoral sediments under raised CO2 and nitrogen supply. Freshwater Biol 48(3):500–511
Lindau CW, DeLaune RD (1991) Dinitrogen and nitrous oxide emission and entrapment in Spartina alterniflora saltmarsh soils following addition of N-15 labelled ammonium and nitrate. Estuar Coast Shelf S 32(2):161–172
Ling Y, Ding H, Xu YT (2008) Effects of reed roots on rhizosphere microbes in constructed wetland. Syst Sci Compre Stud Agr 24(2):214–216
Ludwig B, Teepe R, Lopes de Gerenyu V, Flessa H (2006) CO2 and N2O emissions from gleyic soils in the Russian tundra and a German forest during freeze-thaw periods—a microcosm study. Soil Biol Biochem 38:3516–3519
Lv YH, Bai H, Jiang Y, Yu JH (2008) Study of nitrification activity and impact factors of wetland in Yellow River Delta. Transaction of Oceanol Limno 2:61–66
Magalhães C, Costa J, Teixeira C, Bordalo AA (2007) Impact of trace metals on denitrification in estuarine sediments of the Douro River estuary, Portugal. Mar Chem 107:332–341
Moseman-Valtierra S, Gonzalez R, Kroeger KD, Tang JW, Chao WC, Crusius J, Bratton J, Green A, Shelton J (2011) Short-term nitrogen additions can shift a coastal wetland from a sink to a source of N2O. Atmos Environ 45:4390–4397
Mosier AR (1998) Soil processes and global change. Biol Fert Soils 27:221–229
Mou XJ (2010) Study on the nitrogen biological cycling characteristics and cycling model of tidal wetland ecosystem in Yellow River estuary. Masters Degree Dissertation, Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences, Yantai
Mou XJ, Liu XT, Tong C, Sun ZG (2012) Short-term effects of exogenous nitrogen on CH4 and N2O effluxes from Cyperus malaccensis marsh in the Min River estuary. Environ Sci 33(7):2482–2489
Mou XJ, Sun ZG, Wang LL, Dong HF (2010) Characteristics of nitrogen accumulation and allocation of Suaeda salsa in different growth conditions of intertidal zone in Yellow River estuary. Wetland Sci 8(1):57–66
Muñoz-Hincapié M, Morell JM, Corredor JE (2002) Increase of nitrous oxide flux to the atmosphere upon nitrogen addition to red mangroves sediments. Mar Pollut Bull 44:992–996
Ong Che RG (1999) Concentration of seven heavy metals in soils and mangrove root samples from Mai Po, Hong Kong. Mar Pollut Bull 39:269–79
Priemé A, Christensen S (2001) Natural perturbations, drying-wetting and freezing-thawing cycles, and the emission of nitrous oxide, carbon dioxide and methane from farmed organic soils. Soil Biol Biochem 33:2083–2091
Purvaja R, Ramesh R (2001) Natural and anthropogenic methane emission from coastal wetlands of South India. Environ Manage 27:547–557
Regina K, Nykänen H, Silvola J, Martikainen PJ (1996) Fluxes of nitrous oxide from boreal peatlands as affected by peatland type, water table level and nitrification capacity. Biogeochemistry 35:401–418
Seitzinger SP, Kroeze C (1998) Global distribution of nitrous oxide production and N inputs in freshwater and coastal marine ecosystems. Global Biogeochem Cy 12:93–113
Senga Y, Seike Y, Mchida K, Fujianaga K, Okumura M (2001) Nitrous oxide in Lake Shinji and Nakaumi, Japan. Limnology 2:129–136
Shingo U, Chun-sim UG, Takahito Y (2000) Dynamics of dissolved O2, CO2, CH4 and N2O in a tropical coastal swamp in southern Thailand. Biogeochemistry 49:191–215
Smith CJ, DeLaune RD, Patrick WH Jr (1983) Nitrous oxide emission from Gulf Coast wetlands. Geochim Cosmochim Ac 47(10):1805–1814
Song CC, Zhang JB, Wang YY, Wang YS, Zhao ZC (2008) Emission of CO2, CH4 and N2O from freshwater marsh in Northeast of China. J Environ Manage 88:428–436
Song CC, Zhang LH, Wang YY, Zhao ZC (2006) Annual dynamics of CO2, CH4, N2O emissions from freshwater marshes and affected by nitrogen fertilization. Environ Sci 27(12):2369–2375
Søvik AK, Kløve B (2007) Emission of N2O and CH4 from a constructed wetland in southeastern Norway. Sci Total Environ 380:28–37
Stadmark J, Leonardson L (2007) Greenhouse gas production in a pond sediment: Effects of temperature, nitrate, acetate and season. Sci Total Environ 387(1–3):194–205
State Oceanic Administration of China (2013) Ocean Environmental Quality Communique of China in 2012. (http://www.coi.gov.cn/gongbao/nrhuanjing/nr2012/201304/t20130401_26413.html). Accessed 10 February 2013
Sun LG, Zhu RB, Xie ZQ, Xing GX (2002) Emissions of nitrous oxide and methane from Antarctic Tundra: role of penguin dropping deposition. Atmos Environ 36:4977–4982
Sun ZG, Liu JS, Yang JS, Mou XJ, Wang LL (2009) N2O flux characteristics and emission contributions of Calamagrostis angustiflolia wetland during growth and non-growth seasons. Acta Pratacul Sin 18(6):241–247
Tauchnitz N, Brumme R, Bernsdorf S, Meissner R (2008) Nitrous oxide and methane fluxes of a pristine slope mire in the German National Park Harz Mountains. Plant Soil 303:131–138
Teepe R, Brumme R, Beese F (2001) Nitrous oxide emissions from soil during freezing and thawing periods. Soil Biol Biochem 33:1269–1275
Teiter S, Mander Ü (2005) Emission of N2O, N2, CH4, and CO2 from constructed wetlands from wastewater treatment and from riparian buffer zones. Ecol Eng 25:528–542
Tian JY, Wang XF, Cai XJ (2005) Protection and restoration technique of wetland ecosystem in Yellow River Delta. China Ocean University Press, Qingdao
van Bochove E, Prévost D, Pelletier F (2000) Effects of freeze-thraw and soil structure on nitrous oxide produced in a clay soil. Soil Sci Soc Am J 64:1638–1643
Wan XH, Kuang SF, Zhou HD (2009) Research of exogenous nitrogen input on N2O fluxes in constructed wetland. J China Inst Water Resour Hydropower Res 7(4):264–269
Wang DQ, Chen ZL, Wang J, Xu SY, Yang HX, Chen H, Yang LY, Hu LZ (2007a) Summer-time denitrification and nitrous oxide exchange in the intertidal zone of the Yangtze Estuary. Estuar Coast Shelf S 73:43–53
Wang DQ, Chen ZL, Wang J, Xu SY, Yang HX, Chen H, Yang YL (2007b) Fluxes of CH4, CO2 and N2O fluxes from Yangtze estuary intertidal flat in summer season. Geochimica 36(1):78–88
Wang DQ, Chen ZL, Wang J, Xu SY, Yang HX, Chen H, Yang LY, Hu LZ (2006) Denitrification, nitrous oxide emission and adsorption in intertidal flat, Yangtze estuary, in summer. Geochimica 35(3):271–279
Wang LF, Cai ZC, Yang LF, Meng L (2005) Effect of disturbance and glucose addition on nitrous oxide and carbon dioxide emissions from a paddy soil. Soil Till Res 82:185–194
Wang LL (2011) Study on the nitrous oxide emission rule and influence mechanism of tidal wetland ecosystem in the Yellow River estuary. Masters Degree Dissertation, Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences, Yantai
Wang Q, Liu M, Hou LJ, Cheng SB (2010a) Characteristics and influencing factors of CO2, CH4 and N2O emissions from Chongming eastern tidal flat wetland. Geogr Res 29(5):935–946
Wang ZY, Zhao FF, Zhang BG, Zhang L, Li FM, Gao DM, Luo XX (2010b) Rhizosphere effect of three halophytes in the Yellow River Delta on nitrogen and phosphorus. Environ Sci Technol 33(10):33–38
Whalen SC (2005) Biogeochemistry of methane exchange between natural wetlands and the atmosphere. Environ Eng Sci 22:73–94
World Meteorological Organization (2011) WMO Greenhouse Gas Bulletin No.7 (21 November 2011). http://www.wmo.int/pages/prog/arep/gaw/ghg/documents/GHGbulletin_7_en.pdf. Accessed 15 January 2013
Wrage N, Velthof GL, Laanbroek HJ, Oenema O (2004) Nitrous oxide production in grassland soils: assessing the contribution of nitrifier denitrification. Soil Biol Biochem 36(2):229–236
Xie WX, Zhao QS, Zhang F, Ma XF (2011) Characteristics of N2O flux in estuary wetland of Jiaozhou Bay in autumn and winter. Sci Geogra Sin 31(4):464–469
Xu XG, Guo HH, Chen XL, Lin HP, Du QL (2002) A multi-scale study on land use and land cover quality change: the case of the Yellow River Delta in China. GeoJournal 56:177–183
Yang HX, Wang DQ, Chen ZL, Xu SY (2006) Elementary research on green-house gas emissions in Chongming east intertidal flat of the Changjiang estuary. Mar Environ Sci 25:20–23
Yang P, Tong C, He QH, Huang JF (2012) Diurnal variations of greenhouse gas gluxes at the water-air interface of aquaculture ponds in the Min River estuary. Environ Sci 33(12):4194–4204
Zhang JB, Song CC, Yang WY (2005) Cold season CH4, CO2 and N2O fluxes from freshwater marshes in northeast China. Chemosphere 59(11):1703–1705
Zhang LH, Song CC, Wang DX, Wang YY (2007) Effects of exogenous nitrogen on freshwater marsh plant growth and N2O fluxes in Sanjiang Plain, Northeast China. Atmos Environ 41(5):1080–1090
Zhang YX, Zeng CS, Huang JF, Wang WQ, Tong C (2013) Effects of human-caused disturbance on nitrous oxide flux from Cyperus malaccensis marsh in the Minjiang River estuary. China Environ Sci 33(1):138–146
Zhang YX, Zeng CS, Tong C, Wang WQ, Huang JF, He QH (2012) Tidal action and tread affecting nitrous oxide fluxes in intertidal Cyperus malaccensis var. brevifolius marsh. Ecol Environ Sci 21(4):641–646
Zhu RB, Liu YS, Ma J, Xu H, Sun LG (2008) Nitrous oxide flux to the atmosphere from two coastal tundra wetlands in eastern Antarctica. Atmos Environ 42:2437–2447
Acknowledgments
The authors would like to acknowledge the two anonymous reviewers for their constructive comments on this paper. This study was financially supported by the Strategy Guidance Program of the Chinese Academy of Sciences (no. XD05030404), the “1-3-5” Strategy Plan Program of the Yantai Institute of Coastal Zone Research of the Chinese Academy of Sciences (no. Y254021031), the National Nature Science Foundation of China (no. 41171424), the Key Program of Natural Science Foundation of Shandong Province (no. ZR2010DZ001), the Talents Program of the Youth Innovation Promotion Association, Chinese Academy of Sciences (no. Y129091041), and the Ocean Public Welfare Scientific Research Project, State Oceanic Administration, People's Republic of China (no. 2012418008-3).
Author information
Authors and Affiliations
Corresponding author
Additional information
Resposible editor: Gerhard Lammel
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Sun, Z., Wang, L., Mou, X. et al. Spatial and temporal variations of nitrous oxide flux between coastal marsh and the atmosphere in the Yellow River estuary of China. Environ Sci Pollut Res 21, 419–433 (2014). https://doi.org/10.1007/s11356-013-1885-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-1885-5