Abstract
Purpose
The present study aims to evaluate the effects of intermittent sequential pneumatic compression (ISPC) in the short-term recovery of a repeated sprint interval exercise, including the assessment of power output performance, hematocrit, legs water, and perceptual recovery.
Methods
A randomized, counterbalanced, crossover design was conducted. Sixteen healthy trained individuals (F=7, M=9; 27.7 ± 9.4 years; BMI 22.3 ± 2.9) performed two trials of a cycling fatiguing exercise, followed by a recovery phase (ISPC or Sham), and a subsequent performance assessment exercise to evaluate the effects of ISPC in post-exercise recovery.
Results
There were no significant differences in cycling performance comparing both recovery modes. However, the decrease in the hematocrit levels after the recovery phase was less exacerbated in the ISPC condition compared to Sham (44.03 ± 1.33 vs. 42.38 ± 1.33 %; p = 0.047; d = 0.310). Likewise, the total quality recovery (TQR) was higher after the recovery in the ISPC condition (15.94 ± 0.16 vs. 14.75 ± 0.12 points; p = 0.045; d = 2.125), although no differences were shown previously in power output performance (371.8 ± 22.2 [46.5] vs. 372.4 ± 21.8 [47.2] W; p = 0.986) and rating of perceived exertion (RPE) (17.69 ± 0.41 vs. 17.56 ± 0.31; p = 0.700).
Conclusions
Contrary to our hypothesis, the application of intermittent sequential pneumatic compression after high-intensity exercise reduces the post-exercise hemodilution response and increases perceptual recovery. However, power output was similar between conditions, challenging the effectiveness of this recovery method in a short-term intervention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intermittent sequential pneumatic compression (ISPC) is a therapeutic strategy used in vascular medicine to prevent deep vein thrombosis and reduce the symptoms of lymphedema [1]. This device is thought to provide a mechanical “squeezing” of the limb to move swelling away from the extremity, reducing leg interstitial fluid accumulation in the limbs through the increase in venous return and lymphatic drainage [2]. The sleeves inflate sequentially from distal to proximal through separate chambers, before deflating and repeating this process to provide a ‘milking’ like effect as used in massage [3]. High-intensity exercise causes many physiological changes in the body, including the increase in systemic blood pressure and hemoconcentration [4]. Concomitantly, shifts in water, electrolytes, and metabolites between plasma volume and the extracellular compartment occur [5]. Intermittent sequential pneumatic compression is intended to enhance this physiological process through the stimulation of a rhythmic gradient pressure along the limbs.
Post-exercise blood flow is redistributed causing blood viscosity and hemoconcentration changes in the lower limbs [6]. The removal of edema is induced by a redistribution of skin blood flow, favoring the superficial capillary perfusion [7] and prompting the re-entering of most fluids into the bloodstream through the lymphatic network [8].
Aiming to evaluate the hemoconcentration and the legs water, some studies have chosen sprint interval exercises, which may favor the accumulation of plasma volume in the interstitial space in the legs due to the increase of systemic blood pressure and the subsequent local vasodilation during the recovery between bouts [9, 10].
Hematocrit (Hto) and bioelectrical impedance can be used to evaluate hemoconcentration and segmental legs water content (LWC), respectively. Hematocrit is widely used in exercise assessment, providing information on hemodilution or hemoconcentration. In addition, it is known that acute exercise is associated with an increase in Hto increasing blood viscosity. This increase in Hto is caused because some fluids from the plasma volume are shifting from the bloodstream to the interstitial space, which causes an increase of the proportion of red blood cells within the total volume of plasma. In case of an excessive increase in blood viscosity, the increased wall shear stress may stimulate nitric oxide production by endothelial cells to decrease vascular resistance and increase tissue perfusion by vasodilatory compensation [11]. On the other hand, bioelectrical impedance represents a useful and still underused evaluation tool in lower limb edema formation [12], and it may assess total and segmental water distribution, evaluating the percentage of LWC after high-intensity exercise [13].
To the best of our knowledge, the application of ISPC has shown positive effects on flexibility [14, 15], muscle swelling [16], and lactate removal rate [9], but not on subsequent athletic performance [15, 17] and muscle soreness [18]. The present study aims to compare the effects of ISPC vs. Sham in the post-exercise recovery of repeated sprint exercise, assessing power output performance in Watts (W), Hto, LWC, and perceptual responses to exercise (rating of perceived exertion (RPE) and recovery (total quality recovery (TQR). This study hypothesizes that ISPC enhances the redistribution of locally accumulated flow in higher activity areas, reducing the Hto and the percentage of LWC compared to control and passive recovery placing the same device without the inflation and deflation mode (Sham).
Methods
Participants
An a priori power analysis was conducted using G*Power 3.1.9.7 for F-test (ANOVA: Repeated measures, within-between interaction), using an effect size of 0.3, for 2 groups, and 2 measurements indicated a sample size n = 12 per group at 90% actual power. Then, a sample size of 16 participants was determined to be sufficient to detect a meaningful change. Post-hoc analysis agreed with the preliminary power analysis, with a correlation between repeated measurements higher than 0.85. Sixteen participants were recruited, consisting of 7 females (33.5 ± 10.8 years; 168.1 ± 6.2 cm; 63.1 ± 14.6 kg; BMI 22.2 ± 2.7) and 9 males (26.3 ± 7.5 years; 181.7 ± 7.7 cm; 73.2 ± 7.1 kg; BMI 22.2 ± 3.2). All participants were healthy and trained, regularly engaging in high-intensity interval exercise, including martial arts, team sports, and running with at least three sessions per week.
Design
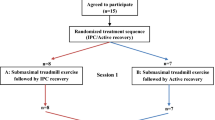
The experimental design of the study is described in Fig. 1. A randomized counterbalanced crossover design was utilized to compare the effectiveness of ISPC in improving post-exercise recovery compared to a passive recovery condition (Sham).
Participants reported to the University research laboratory on two separate occasions. They completed two trials of exercise, recovery, and exercise to evaluate the effect of ISPC in post-exercise recovery and subsequent performance. During the recovery protocol, participants were randomly assigned to either ISPC or a Sham recovery condition using simple randomization. The visits were scheduled seven days apart, at the same time and day of the week, to minimize any potential diurnal variations. Participants were instructed to arrive in a rested, hydrated, postprandial state (> 2 h), having abstained from caffeine, alcohol, and strenuous exercise in the 24 h preceding a session. Water intake was not allowed during the experimental procedures. They were also advised to maintain normal dietary habits throughout the study and replicate their 24 h diet for subsequent visits.
Procedures
Participants were described for height, weight, and body fat at the beginning of the study. Baseline measures of Hto and LWC were collected after 5 min of a resting period in a horizontal position. Following baseline measurements, the cycle ergometer seat height and position were adjusted for each subject before starting the exercise. Exercise protocol 1, consisted of 8 sets of 20 s to maximal with 60 s of passive recovery between sets. After the exercise, participants rated their perceived exertion on the RPE scale, and a second measurement of Hto and LWC was taken before starting the recovery phase. The RPE scale ranged from 6 (very, very light) to 20 (very, very hard) [19]. In the recovery phase, either the ISPC or the Sham condition was applied for 30 min in a resting, supine position. Participants were informed that leg sleeves could be activated or not. The ISPC condition provided 30 min of pneumatic compression, while the Sham condition involved 30 min of an equally resting and supine position with the ISPC device placed in a deflated mode, as has been done in other studies [9, 20]. This protocol was employed to control for any thermogenic effect of wearing the legs sleeves as heat loss from the legs is likely relevant during the 30-min recovery phase. Another measurement of Hto and LWC was taken at the end of the recovery phase, and participants rated their perceived recovery on the TQR scale before starting exercise protocol 2. The TQR scale ranged from 6 (very, very poor recovery) to 20 (very, very good recovery) [21]. Then, exercise protocol 2 consisted of 3 sets of 20 s to maximum with 60 s of passive recovery between sets to evaluate cycling performance and the effectiveness of ISPC during the recovery.
Hematocrit
Hematocrit concentrations were measured to evaluate the blood viscosity in relation to exercise and recovery. Two samples were taken from the earlobe, punching with a safety lancet and collecting the blood with a microhematocrit capillary tube before the first exercise, immediately after the first exercise, and after recovery, to obtain a reliable measure. The position was standardized for all the Hto measurements in a seated position. Hematocrit was determined by centrifuging the blood for 8 min at 8000 rpm and 1000 g. A blinded technician read the samples in a randomized manner to avoid bias in the lectures. An additional cardiovascular assessment during the post-exercise recovery of the experiment is presented by Artés et al. [22].
Bioelectrical impedance
Bioelectrical impedance (bioelectrical impedance analysis; multi-frequency segmental body composition analyzer (MC-780U; Tanita, Tokyo Japan) was used for the measurement of body water composition. It is based on the ability of biological tissue to impede electric current the so-called resistance which is caused by total body water and reactance. The segmental LWC was measured by direct segmental multi-frequency impedance analysis which has been correlated with edema swelling [23]. In this sense, bioelectrical impedance has been previously used to provide sensitive information on lower limb edema [13, 24, 25].
Exercise protocol 1
All testing was performed on the same cycle ergometer (Concept2, Inc., Morrisville, VT, USA). The repeated sprint exercise consists of a training named sprint interval training (SIT), which is a type of training that consists of a short period of exertion (≤ 30 s) of near maximal (all-out) intensity, equal or less to 12 sets [26, 27]. Aiming to evaluate the legs water and the hemoconcentration, it was considered that this kind of exercise would favor the accumulation of fluid volume in the interstitial space of the legs due to the increase of systemic blood pressure by maximum and short exertion, and due to the secondary local vasodilation during the recovery between bouts of exercise. The warm-up protocol consisted of a warm-up of 5 min at 1 W/kg, followed by 3 min at 1.5 W/kg and 2 min at 2 W/kg. Then, the main exercise consists of 8 sets of 20 s to maximal with 60 s of passive recovery between sets. The damper setting of this bicycle works on a scale from 1 to 10, which can be modified using the lever on the side of the flywheel cage. The warm-up was performed with a level of 4, always that a minimum of 70 revolutions per minute was established. During the sprint repetitions, a damper setting of 8 was selected. After the bout of exercise, there was no cool down to avoid an active recovery phase and participants went directly to lie down on the stretcher to receive the randomized recovery procedure.
The only instruction given to participants was to perform the entire exertion as maximally as possible. Power output performance was assessed during the repeated sprint exercise. The number of W was blindly monitored in every subject and every sprint performed. At exactly 5 min after the end of the exercise, the ISPC or Sham recovery method began. Ratings of perceived exertion (Borg’s 6–20 scale) were asked at the end of the exercise.
Intermittent sequential pneumatic compression
An intermittent sequential pneumatic compression device (Recovery Air 3 PRO, Therabody, Los Angeles, CA, USA) was used for the ISPC condition. The ISPC device consists of 2 separate “leg sleeves” which contain 4 circumferential inflatable chambers (arranged linearly along the limb) encompassing the leg from the feet to the hip. The “leg sleeves” are connected to an automated pneumatic pump at which target inflation pressures for each zone and the duty cycle can be controlled. The compression modality used was ‘sequential’, where pneumatic pressure is applied to parts of the limb in sequence (normally distal to proximal) with 4 overlapped chambers per sleeve, creating a negative gradient of 80, 79, 78, and 77 mmHg from distal to proximal. Each cycle consisted of inflating and deflating completely the sleeves once per minute for 30 min. Upon completion of the recovery period, the sleeves were cleaned and disinfected to remove sweat from each participant. After two minutes following the recovery period, the participant was seated on the ergometer ready for cycling bout two.
Exercise protocol 2
After the recovery phase, another bout of exercise consisting of 3 sets of 20 s to maximal with 60 s of recovery between sets was performed to evaluate cycling performance after a fatiguing exercise and an acute recovery modality. Participants were instructed to do the exercise as maximum they could just after the measurement in the body composition analyzer to assess cycling performance.
Statistical analysis
Statistical analyses were performed using the Statistical Package for Social Science (V. 25.0, SPSS Inc., Chicago, IL). Descriptive statistics are shown as means ± standard error of the mean [95% CI]. The normality of the data for all measures was confirmed by the Kolmogorov-Smirnoff test. A two-way repeated measures ANOVA was used to determine differences between groups (ISPC and Sham) and time (pre, post-exercise, and post-recovery intervention) for change in power output performance (W), LWC, Hto, RPE, and TQR. In the case of detecting statistical effects (p < 0.05), Bonferroni comparisons were performed. Effect size (Cohen d) was calculated to estimate the magnitude of the difference between group means, with d = 0.1, 0.3, 0.5, 0.7, and 0.9 reflecting small, medium, large, very large, and extremely large effect sizes, respectively.
Results
The order of the interventions (ISPC and Sham) was considered in the statistical model, and no statistically significant differences were found regarding the order of the interventions.
Performance
Figure 2 shows the evaluation of the power output performance (W) between ISPC and Sham conditions during the exercise protocols. For exercise protocol 1, the test re-test intraclass correlation between the exercises performed in both conditions (ICC 3, 1) was 0.976 before starting the recovery phase.
During exercise protocol 1, no significant differences were observed between the ISPC and Sham conditions in mean power output from the 8 sprints (371.8 ± 22.2 [46.5] vs. 372.4 ± 21.8 [47.2] W; p = 0.986). Relative mean power output was 5.08 and 5.09 W/kg, respectively. The comparison between repetition 1 vs. 8 shows a decrease of 18.8 % for ISPC and 17.7 % for Sham condition.
In exercise protocol 2, there were no significant differences between conditions, respectively ISPC and Sham (400.1 ± 27.3 [58.2] vs. 398.3 ± 24.6 [52.3] W; p = 0.845). Relative mean power output was 5.46 and 5.44 W/kg, respectively.
Hematological changes
Baseline measures of Hto show a test re-test intraclass correlation between conditions (ICC 3, 1) of 0.850. The average of the three Hto measurements was not statistically significant different between ISPC and Sham conditions, but a moderate effect was observed (46.14 ± 1.33 vs. 45.23 ± 1.57 %; p = 0.060; d = 0.625; Fig. 3A). When comparing the end of exercise 1 and the end of the recovery phase, both ISPC and Sham conditions resulted in a decrease in Hto (49.06 ± 1.28 vs. 43.2 ± 1.28 %; p = 0.007; d = 4.578). Pairwise comparisons revealed that the ISPC condition had a significantly smaller decrease in post-recovery Hto compared to Sham after the recovery protocol (44.03 ± 1.33 vs. 42.38 ± 1.33 %; p = 0.047; d = 0.310; Fig. 3A), expressing a closer return to baseline values for the ISPC condition and a pronounced decrease in the hemoconcentration in the Sham condition.
Changes in hematocrit A and legs water content B from basal to post-exercise and post-recovery in 16 healthy trained participants. Data shows the comparison between ISPC (dotted lines, hollow symbols) and Sham (solid line; filled symbols). Significant differences between the two conditions (p < 0.05) are denoted by an asterisk
Legs water content
Baseline measures of the LWC show a test re-test intraclass correlation between conditions (ICC 3, 1) of 0.988. There were no significant differences between ISPC and Sham conditions at baseline (28.67 ± 0.78 vs. 28.72 ± 0.79 %; p = 0.738; Fig. 3B), at the end of the exercise (28.95 ± 0.78 vs. 28.89 ± 0.79; p = 0.647; Fig. 3B), and after the recovery phase (28.72 ± 0.79 vs. 28.47 ± 0.82; p = 0.071; d = 0.310; Fig. 3B). However, the comparison between conditions at the end of the recovery phase shows a moderate effect on the intervention. This suggests that ISPC could favor the reduction of legs compartmental water in both lower extremities after this recovery mode.
The potential correlation between delta changes in LWC and Hto from post-exercise 1 to post-recovery was evaluated by a Pearson correlation. However, there were no statistically significant associations in either ISPC (r = 0.185, p = 0.493) or Sham condition (r = 0.018, p = 0.948).
Total quality recovery (TQR) and rating of perceived exertion (RPE)
The study found that there were no significant differences between ISPC and Sham conditions in the RPE after exercise protocol 1 (17.69 ± 0.41 [0.87] vs. 17.56 ± 0.31 [0.67]; p = 0.700) nor after exercise protocol 2 (14.94 ± 0.45 [0.96] vs. 15.38 ± 0.43 [0.77]; p = 0.184; Fig. 4), which indicates that both groups perceived the same exhaustion during the exercises. When comparing exercise protocols 1 and 2, a decrease of 2.47 ± 0.52 points (p < 0.001) was observed in the perception of fatigue in both groups, showing a higher perceptual exertion after the first and longer set of exercise. In terms of TQR, there was a significant difference between ISPC and Sham conditions after the recovery protocol, with ISPC showing a higher perception of recovery compared to Sham (15.94 ± 0.16 [1.34] vs.14.75 ± 0.12 [1.02]; p = 0.045; d = 2.125). This shows that ISPC was more effective in promoting perceived recovery after exercise compared to Sham intervention.
Violin plots showing the changes in the rating of perceived exertion after exercise protocol 1 (RPE-1), the total quality recovery after the recovery phase (TQR), and the rating of perceived exertion after exercise protocol 2 (RPE-2) in 16 healthy trained participants. Data shows the comparison between ISPC (hollow areas) and Sham (grey-filled areas) conditions. The median is shown with a dashed line and the 1st and 3rd quartiles with dotted lines. Significant differences between the two conditions (p < 0.05) are denoted by an asterisk
Discussion
The results of this study indicate that the use of ISPC following high-intensity sprint interval exercise reduces post-exercise hemodilution and improves perceptual recovery, without significant effects on LWC and subsequent cycling performance. Our initial hypothesis was that ISPC would induce a faster recovery of the exercise-induced rise in hemoconcentration (reflecting an increase in plasmatic volume due to the mobilization of previously accumulated fluids in the limbs ), while Sham would have still a hemoconcentration higher than basal after the passive recovery. However, we found that the Sham condition had a more abrupt hemodilution response below baseline levels, while the ISPC condition showed a more accurate recovery of baseline hemoconcentration parameters after a fatiguing bout of exercise.
To date, some studies have investigated the effects of ISPC on cycling performance after maximal intensity exercise. Our study aligns with the findings of O’Donnell and Driller [3] who found that there were no significant differences between ISPC and control conditions in triathlon performance and blood lactate concentration. In the same direction, Martin et al. [9] showed no significant differences in peak power, average power, and fatigue index in a healthy and active group of participants. However, they observed that blood lactate was significantly lower at 25 and 35 min of recovery for ISPC vs. Sham.
On the other hand, Haun et al. [20] reported a significant improvement in a time-to-exhaustion cycling test following the use of a modified sequential pneumatic compression device for 1 h when compared to passive recovery. These results are consistent with other studies that suggest that ISPC may have positive effects on neuromuscular performance and heart rate recovery immediately after the recovery application [18, 28, 29]. Wiener et al. [28], found that using ISPC as a treatment of fatigued muscles after a sustained exertion and provided higher mean power frequency, which is a well-established indicator at high levels of muscle activity, in comparison to passive recovery. Likewise, peak power and accumulative peak power increased by 11% and 14% respectively throughout the trial [29].
Interestingly, these studies used longer bouts of exercise to evaluate the effect of ISPC on recovery and performance. In our study, participants showed similar mean power (W) in both conditions, indicating a lack of significant effects in consecutive cycling performance.
Regarding the changes in leg edema during the intervention, and segmental LWC and Hto were evaluated. It is known that the rate of recovery of blood hemoconcentration may depend on the intensity and duration of the exertion [30], therefore at the end of the exercise, fluid accumulates in the muscles and the interstices [31]. After exercise protocol 1, all participants had an augmented hemoconcentration (Fig. 3A) indicating that a high-intensity exercise was performed. The physiological underlying mechanisms are related to a set of factors such as venous obstruction, increased capillary permeability, or increased plasma volume secondary to sodium and water retention during exercise [32]. Khan et al. [33] also showed that heart rate recovery was higher when applying an 80 mmHg ISPC in a supine position after a submaximal running exercise. Some authors have described that ISPC could increase blood flow, venous return, cardiac output, and blood pressure and produce hyperemia up to 40 s after the release of the compression [34, 35]. Interestingly, we found a decrease in Hto on the Sham condition, meanwhile, the ISPC condition had a higher hemoconcentration after the recovery phase, being closer to baseline parameters. Further studies should evaluate the unexpected higher post-exercise hemodilution in the Sham condition (below baseline), and its potential implications in the athletic recovery.
Regarding LWC, we did not find significant differences between ISPC and Sham in the percentage of water in the lower extremities after the recovery phase (p = 0,071). However, ISPC had a moderate effect (d = 0.310; Fig. 3B), indicating that they could remove faster interstitial fluid accumulation during the recovery. A larger sample size may be needed to support this finding. As has been shown, ISPC may prevent the formation of fluid in the interstitial space [36] and could generate drainage of intracellular water towards the vessels in relation to the localized swelling caused by plasmatic accumulation [16], which could explain our results. The results obtained give us indications that the ISPC helps to reduce the volume of legs water, which suggests that it may help to send more fluid from the muscle to the vessels [35].
Regarding TQR, our analysis showed an increase in perceptual recovery after the recovery phase in the ISPC condition, while RPE was similar after both, the first and second exercise bouts. The perceptual recovery was described as reduced stiffness and comfort, suggesting that fluids may have been mobilized in a thixotropic manner. The increased perceptual recovery is aligned with other authors, who found that ISPC could reduce subjective muscular fatigue scores with immediate subjective benefit acutely and after 24 h [37, 38] and a reduction of muscle tenderness and muscle stiffness [15]. On the contrary, other studies showed only a trend towards a positive effect of the ISPC over the TQR after an interval exercise of longer duration than our protocol [3, 18] and others did not obtain significant differences in relation to TQR or blood lactate concentration [39].
Mechanisms associated with improved perceived recovery remain unclear. Despite the differences found between studies in relation to the objective parameters, it must be taken into consideration that the level of fatigue perception is an essential psychological component to face an exertion, therefore, not only increasing performance but improving perceptual recovery may be beneficial by influencing the athlete’s decision-making on subsequent exertions. Providing subjective ratings of recovery (TQR) when not blinded to the treatment is subject to potential placebo effects. Therefore, the increases in perceived recovery with ISPC must be taken with caution in comparison with passive supine recovery. The placebo effect has neurobiological underpinnings and actual effects on the brain and body [40], which are based on classical conditioning and expectancy [41]. Devices such as ISPC are supported by wellness companies in social media which can affect the belief of the participant. However, despite the increase in TQR after the fatiguing exercise 1 after using ISPC, there were similar mean power output in exercise 2, which could suggest that ISPC could have a placebo effect on the athlete's psychology, without relevant impact on subsequent physiological performance.
It is relevant to acknowledge the limitations of this study to provide a comprehensive interpretation of the findings. In this case, the authors recognize that the lack of assessment of fatigue and potential recovery 24–72 h after the exertion is a relevant limitation. This factor is interesting given that the perception of short-term recovery may be biased by using the material, causing a placebo effect in the participants with the ISPC recovery protocol in the short-term recovery. The authors also note that muscle damage biomarkers were not assessed, which may have impacted participants’ baseline and post-exercise status. Finally, there was no comparison with post-exercise active recovery, which provides a skeletal muscle pump for vasodilated vasculature, also being a natural and freely accessible recovery method to improve hemodynamic recovery after high-intensity exercise. Despite these limitations, the study provides interesting insights into the potential benefits of ISPC on short-term physiological and perceptual recovery in athletes.
Conclusions
The application of intermittent sequential pneumatic compression after a sprint interval exercise may have beneficial effects on recovery. Specifically, the use of ISPC reduces the exacerbated hemodilution response observed at the end of the high-intensity exercise and improves perceptual recovery in comparison with passive recovery. However, ISPC does not improve power output performance in a subsequent exercise, thus challenging the short-term effectiveness of this recovery method. These results may have significant implications for enhancing readiness and training tolerance in competitive sports. Further studies in ISPC should include an ‘active recovery group’ when hemodynamic measurements are collected to provide a more valid ecological method.
Data availability
No datasets were generated or analysed during the current study.
References
Morris RJ (2008) Intermittent pneumatic compression—systems and applications. J Med Eng Technol. https://doi.org/10.1080/03091900601015147
Born DP, Sperlich B, Holmberg HC (2013) Bringing light into the dark: effects of compression clothing on performance and recovery. Int J Sports Physiol Perform. 8(1):4–18
O’Donnell S, Driller MW (2015) The effect of intermittent sequential pneumatic compression on recovery between exercise bouts in well-trained triathletes. J Sci Cycling 4:24–8
Komka Z, Szilágyi B, Molnár D, Sipos B, Tóth M, Sonkodi B et al (2022) Exercise-related hemoconcentration and hemodilution in hydrated and dehydrated athletes: an observational study of the Hungarian canoeists. PLoS ONE. https://doi.org/10.1371/journal.pone.0277978
Chatard JC, Atlaoui D, Farjanel J, Louisy F, Rastel D, Guézennec CY (2004) Elastic stockings, performance and leg pain recovery in 63 year-old sportsmen. Eur J Appl Physiol. 93(3):347–52. https://doi.org/10.1007/s00421-004-1163-9
Varlet-Marie E, Brun JF, Raynaud de Mauverger E, Fédou C (2017) Exercise-induced changes in hematocrit and hematocrit/viscosity ratio in male rugby players. Clin Hemorheol Microcirc. 64(4):817–26. https://doi.org/10.3233/CH-168042
Malanin Pertti J, Kolari Väinö KHK. (1999) The role of low resistance blood flow pathways in the pathogenesis and healing of venous leg ulcers. Acta Derm Venereol 79(2):156–60
Levick JR, Michel CC (2010) Microvascular fluid exchange and the revised starling principle. Cardiovasc Res. 87(2):198–210. https://doi.org/10.1093/cvr/cvq062
Martin JS, Friedenreich ZD, Borges AR, Roberts MD (2015) Acute effects of peristaltic pneumatic compression on repeated anaerobic exercise performance and blood lactate clearance. J strength cond res. 29(10):2900–2906
Watt K, Hopkins W, Snow R (2002) Reliability of performance in repeated sprint cycling tests. J Sci Med Sport. 5(4):354–61
Connes P, Simmonds MJ, Brun JF, Baskurt OK (2013) 2013 Exercise hemorheology: classical data, recent findings and unresolved issues. Clin Hemorheol Microcirc 53(1–2):187–99. https://doi.org/10.3233/CH-2012-1643
Gianesini S, Raffetto JD, Mosti G, Maietti E, Sibilla MG, Zamboni P et al (2020) Volume control of the lower limb with graduated compression during different muscle pump activation conditions and the relation to limb circumference variation. J Vasc Surg Venous Lymphat Disord. 8(5):814–20
Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM et al (2004) Bioelectrical impedance analysis–part I: review of principles and methods. Clin Nutr. 23(5):1226–43
Sands WA, McNeal JR, Murray SR, Stone MH (2015) Dynamic compression enhances pressure-to-pain threshold in elite athlete recovery. J Strength Cond Res. 29(5):1263–72
Sands WA, Murray MB, Murray SR, McNeal JR, Mizuguchi S, Sato K et al (2014) Peristaltic pulse dynamic compression of the lower extremity enhances flexibility. J Strength Cond Res. 28(4):1058–64
Chleboun GS, Howell JN, Baker HL, Ballard TN, Graham JL, Hallman HL et al (1995) Intermittent pneumatic compression effect on eccentric exercise-induced swelling, stiffness, and strength loss. Arch Phys Med Rehabil. 76(8):744–9
Martin JS, Friedenreich ZD, Borges AR, Roberts MD (2015) Preconditioning with peristaltic external pneumatic compression does not acutely improve repeated wingate performance nor does it alter blood lactate concentrations during passive recovery compared with sham. Appl Physiol Nutr Metab 40(11):1214–7. https://doi.org/10.1139/apnm-2015-0247
Draper SN, Kullman EL, Sparks KE, Little K, Thoman J (2020) Effects of intermittent pneumatic compression on delayed onset muscle soreness (DOMS) in long distance runners. Int J Exerc Sci. 13(2):75–86
Borg GA (1982) Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 14(5):377–81
Haun CT, Roberts MD, Romero MA, Osburn SC, Mobley CB, Anderson RG et al (2017) Does external pneumatic compression treatment between bouts of overreaching resistance training sessions exert differential effects on molecular signaling and performance-related variables compared to passive recovery? Exploratory study. PLoS ONE. 12(6):e0180429. https://doi.org/10.1371/journal.pone.0180429
Kenttä G, Hassmén P (1998) Overtraining and recovery. Sports Med 26(1):1–16. https://doi.org/10.2165/00007256-199826010-00001
Artés A, Ferrer-Ramos P, Javierre C, Viscor G, García I (2024) Effects of intermittent pneumatic compression on the recovery of cardiovascular parameters after repeated sprint exercise. Eur J Appl Physiol. 124(4):1037–1048. https://doi.org/10.1007/s00421-023-05333-x
Toshima M, Morino Y (2022) Water distribution changes in complex decongestive treatment for leg lymphedema: quantitative evaluation by direct segmental multi-frequency bioimpedance analysis. Ann Vasc Dis. 15(2):94–100
Barbosa EJL, Glad CAM, Nilsson AG, Bosaeus N, Nyström HF, Svensson PA et al (2014) Extracellular water and blood pressure in adults with growth hormone (gh) deficiency: a genotype-phenotype association study. PLoS ONE 9(8):e105754. https://doi.org/10.1371/journal.pone.0105754
Matias CN, Santos DA, Júdice PB, Magalhães JP, Minderico CS, Fields DA et al (2016) Estimation of total body water and extracellular water with bioimpedance in athletes: a need for athlete-specific prediction models. Clin Nutr 35(2):468–74
Sloth M, Sloth D, Overgaard K, Dalgas U (2013) Effects of sprint interval training on VO2max and aerobic exercise performance: a systematic review and meta-analysis. Scand J Med Sci Sports. 23(6):e341-52
Jiménez-Maldonado A, García-Suárez PC, Rentería I, Moncada-Jiménez J, Plaisance EP (2020) Impact of high-intensity interval training and sprint interval training on peripheral markers of glycemic control in metabolic syndrome and type 2 diabetes. Biochimica Et Biophysica Acta Mol Basis Dis 1866:165820
Wiener A, Mizrahi J, Verbitsky O (2001) Enhancement of tibialis anterior recovery by intermittent se-quential pneumatic compression of the legs. Basic Appl Myol. 11(2):87–89
Roberts LA, Caia J, James LP, Scott TJ, Kelly VG (2019) Effects of external counterpulsation on postexercise recovery in elite rugby league players. Int J Sports Physiol Perform. 14(10):1350–6
Harrison MH (1985) Effects on thermal stress and exercise on blood volume in humans. Physiol Rev. 65(1):149–209. https://doi.org/10.1152/physrev.1985.65.1.149
Sjogaard G, Saltin B (1982) Extra-and intracellular water spaces in muscles of man at rest and with dynamic exercise. Am J Physiol Regul Integr Comp Physiol 243(3):R271-80. https://doi.org/10.1152/ajpregu.1982.243.3.R271
O’Brien JG, Chennubhotla SA, Chennubhotla RV (2005) Treatment of edema. Am Fam Physician. 71(11):2111–2117
Khan Z, Ahmad I, Hussain ME (2021) Intermittent pneumatic compression changes heart rate recovery and heart rate variability after short term submaximal exercise in collegiate basketball players: a cross-over study. Sport Sci Health. 17(2):317–26. https://doi.org/10.1007/s11332-020-00684-w
Fanelli G, Zasa M, Baciarello M, Mazzani R, Di Cianni S, Rossi M et al (2008) Systemic hemodynamic effects of sequential pneumatic compression of the lower limbs: a prospective study in healthy volunteers. J Clin Anesth. 20(5):338–42
Lattimer CR, Kalodiki E, Azzam M, Geroulakos G (2015) Pneumatic thigh compression reduces calf volume and augments the venous return. Phlebology. 30(5):316–22
Zelikovski A, Kaye CL, Fink G, Spitzer SA, Shapiro Y (1993) The effects of the modified intermittent sequential pneumatic device (MISPD) on exercise performance following an exhaustive exercise bout. Br J Sports Med. 27(4):255–9. https://doi.org/10.1136/bjsm.27.4.255
Heapy AM, Hoffman MD, Verhagen HH, Thompson SW, Dhamija P, Sandford FJ et al (2018) A randomized controlled trial of manual therapy and pneumatic compression for recovery from prolonged running—an extended study. Res Sports Med 26(3):354–64. https://doi.org/10.1080/15438627.2018.1447469
Cranston AW, Driller MW (2022) Investigating the use of an intermittent sequential pneumatic compression arm sleeve for recovery after upper-body exercise. J Strength Cond Res. 36(6):1548–53. https://doi.org/10.1519/JSC.0000000000003680
Overmayer RG, Driller MW (2018) Pneumatic compression fails to improve performance recovery in trained cyclists. Int J Sports Physiol Perform 13(4):490–5. https://doi.org/10.1123/ijspp.2017-0207
Szabo A (2013) Acute psychological benefits of exercise: reconsideration of the placebo effect. J Mental Health 22(5):449–55. https://doi.org/10.3109/09638237.2012.734657
Stewart-Williams S, Podd J (2004) The placebo effect: dissolving the expectancy versus conditioning debate. Psychol Bull 130(2):324–40. https://doi.org/10.1037/0033-2909.130.2.324
Acknowledgements
The authors would like to thank all the participants for their time and commitment in undertaking this study and Therabody Corp® for facilitating the devices to conduct the study.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception, design, and review of the final version of the manuscript. P.F.R.: investigation, data curation, writing—the original manuscript; A.A.: investigation, data curation, and writing—the original manuscript; C.J.: conceptualization, formal analysis, resources, writing—review & editing; G.V.: supervision, project administration, resources, writing—review & editing; I.G.: conceptualization, methodology, formal analysis, writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
IG has been a freelance consultant for Therabody Corp® between 2020 and 2022. The work described here is solely reflective of the author’s personal views and is unrelated to his relationship with Therabody Corp®. Likewise, Therabody Corp®. had no role in the conception, writing, revision, or final approval of the manuscript. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be interpreted as a potential conflict of interest.
Ethical approval
The study was carried out according to the Declaration of Helsinki for human experimentation and was approved by the Institutional Ethical Committee from the University of Barcelona (Institutional Review Board no. IRB00003099).
Informed consent
All participants were informed about the study and received an information sheet, then, they gave written consent before taking part in the study and were free to withdraw from the experimental protocol at any time.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ferrer-Ramos, P., Artés, A., Javierre, C. et al. Intermittent sequential pneumatic compression reduces post-exercise hemodilution and enhances perceptual recovery without improving subsequent cycling performance. Sport Sci Health (2024). https://doi.org/10.1007/s11332-024-01217-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11332-024-01217-5