Abstract
Background
Astrocytes as the most abundant brain cells play a variety of critical roles in neuroplasticity and normal brain function. Many epileptic patients show changes in astroglia morphology and function named reactive astrogliosis. The beneficial role of exercise has been reported in several studies. This study aimed to assess the effect of regular moderate exercise on astrocytes alteration followed by chronic seizures in the somatosensory cortex.
Methods
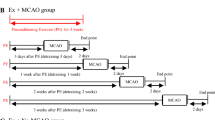
Male Wistar rats were divided into five groups: sham, pentylenetetrazole (PTZ), exercise (EX), EX before PTZ and EX + PTZ. Animals in the sham group received saline every other day for 4 weeks intraperitoneally (i.p.). Chronic seizures were induced by an i.p. injection of PTZ (35 mg/kg) every other day for 4 weeks. The protocol of exercise was running on a treadmill for 30 min/day 5 days a week at a mild intensity. The mean percentage of GFAP, C3 and S100A10 reacted astrocytes in the somatosensory cortex was assessed using immunohistochemistry.
Findings.
Our findings indicated that GFAP, as well as S100A10 expression, increased in the EX and the EX before PTZ group. The expression of the C3 receptor as a marker of neurotoxic astrocytes decreased in the three groups of exercise. In addition, the C3/S100A10 ratio in the somatosensory cortex decreased in the three groups of exercise.
Conclusion
Exercise would remodel astrocytes in the somatosensory cortex in favor of producing more neuroprotective astrocytes following the chronic seizure. Further, the improvement of neuroprotective astrocytes may be involved in the antiepileptogenesis effect of preconditioning exercise.




Similar content being viewed by others
References
Abbott NJ, Rönnbäck L, Hansson E (2006) Astrocyte–endothelial interactions at the blood–brain barrier. Nat Rev Neurosci 7(1):41–53
Liddelow S, Barres B (2015) SnapShot: astrocytes in health and disease. Cell 162 (5):1170–1170. e1171
Shigetomi E, Saito K, Sano F, Koizumi S (2019) Aberrant calcium signals in reactive astrocytes: a key process in neurological disorders. Int J Mol Sci 20(4):996
Zhou B, Zuo YX, Jiang RT (2019) Astrocyte morphology: Diversity, plasticity, and role in neurological diseases. CNS Neurosci Ther 25(6):665–673
Losi G, Cammarota M, Carmignoto G (2012) The role of astroglia in the epileptic brain. Front Pharmacol 3:132
Miller SJ (2018) Astrocyte heterogeneity in the adult central nervous system. Front Cell Neurosci 12:401
Pekny M, Wilhelmsson U, Pekna M (2014) The dual role of astrocyte activation and reactive gliosis. Neurosci Lett 565:30–38
Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung W-S, Peterson TC (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541(7638):481–487
Li T, Liu T, Chen X, Li L, Feng M, Zhang Y, Wan L, Zhang C, Yao W (2020) Microglia induce the transformation of A1/A2 reactive astrocytes via the CXCR7/PI3K/Akt pathway in chronic post-surgical pain. J Neuroinflammation 17(1):1–15
Li T, Chen X, Zhang C, Zhang Y, Yao W (2019) An update on reactive astrocytes in chronic pain. J Neuroinflammation 16(1):1–13
Wasterlain CG, Chen JW (2008) Mechanistic and pharmacologic aspects of status epilepticus and its treatment with new antiepileptic drugs. Epilepsia 49:63–73
Acharya MM, Hattiangady B, Shetty AK (2008) Progress in neuroprotective strategies for preventing epilepsy. Prog Neurobiol 84(4):363–404
Barzroodi Pour M, Bayat M, Navazesh A, Soleimani M, Karimzadeh FJNR (2021) Exercise Improved the Anti-Epileptic Effect of Carbamazepine through GABA Enhancement in Epileptic Rats 46(8):2112–2130
Howard GM, Radloff M, Sevier TLJCsmr (2004) Epilepsy and sports participation. 3 (1):15–19
Setkowicz Z, Mazur A (2006) Physical training decreases susceptibility to subsequent pilocarpine-induced seizures in the rat. Epilepsy Res 71(2–3):142–148
Nakken K, Bjørholt P, Johannessen S, LoSyning T, Lind E (1990) Effect of physical training on aerobic capacity, seizure occurrence, and serum level of antiepileptic drugs in adults with epilepsy. Epilepsia 31(1):88–94
Arida RM, Scorza FA, Terra VC, Scorza CA, de Almeida A-C, Cavalheiro EA (2009) Physical exercise in epilepsy: what kind of stressor is it? Epilepsy Behav 16(3):381–387
Zavvari F, Alivan F, Abdi M, Jahanbazi Jahan-Abad A, Karimzadeh FJSSfH (2022) Maternal exercise during pregnancy increases neuregulin-1 and ErbB4 expression in the newborn offspring of Wistar rats.1–7
Albrecht H (1986) Endorphins, sport and epilepsy: getting fit or having one? N Z Med J 99(814):915
Barzroodi Pour M, Bayat M, Golab F, Eftekharzadeh M, Katebi M, Soleimani M, Karimzadeh F (2019) The effect of exercise on GABA signaling pathway in the model of chemically induced seizures. Life sciences 232:116667. doi:https://doi.org/10.1016/j.lfs.2019.116667
Holmes PV, Reiss JI, Murray PS, Dishman RK, Spradley JM (2015) Chronic exercise dampens hippocampal glutamate overflow induced by kainic acid in rats. Behav Brain Res 284:19–23
Kim H-j, Kim I-K, Song W, Lee J, Park S (2013) The synergic effect of regular exercise and resveratrol on kainate-induced oxidative stress and seizure activity in mice. Neurochem Res 38(1):117–122
Lundquist AJ, Parizher J, Petzinger GM, Jakowec MW (2019) Exercise induces region-specific remodeling of astrocyte morphology and reactive astrocyte gene expression patterns in male mice. J Neurosci Res 97(9):1081–1094
Li F, Geng X, Yun HJ, Haddad Y, Chen Y, Ding Y (2021) Neuroplastic Effect of Exercise Through Astrocytes Activation and Cellular Crosstalk. Aging Dis 12(7):1644–1657
Tatsumi K, Okuda H, Morita-Takemura S, Tanaka T, Isonishi A, Shinjo T, Terada Y, Wanaka A (2016) Voluntary exercise induces astrocytic structural plasticity in the globus pallidus. Front Cell Neurosci 10(165):1–12
Barzroodi Pour M, Bayat M, Navazesh A, Soleimani M, Karimzadeh F (2021) Exercise Improved the Anti-Epileptic Effect of Carbamazepine through GABA Enhancement in Epileptic Rats. Neurochem Res 46(8):2112–2130
Bayat M, Golab F, Eftekharzadeh M, Katebi M, Soleimani M, Karimzadeh F (2019) The effect of exercise on GABA signaling pathway in the model of chemically induced seizures. Life Sci 232(116667):1–9
Aronica E, Boer K, Van Vliet E, Redeker S, Baayen J, Spliet W, Van Rijen P, Troost D, Da Silva FL, Wadman W (2007) Complement activation in experimental and human temporal lobe epilepsy. Neurobiol Dis 26(3):497–511
Coulter DA, Steinhäuser C (2015) Role of astrocytes in epilepsy. Cold Spring Harb Perspect Med 5(3):a022434
Steinhäuser C, Grunnet M, Carmignoto G (2016) Crucial role of astrocytes in temporal lobe epilepsy. Neuroscience 323:157–169
Wilhelmsson U, Bushong EA, Price DL, Smarr BL, Phung V, Terada M, Ellisman MH, Pekny M (2006) Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc Natl Acad Sci 103(46):17513–17518
Middeldorp J, Hol E (2011) GFAP in health and disease. Prog Neurobiol 93(3):421–443
Li J, Ding Y-H, Rafols JA, Lai Q, McAllister JP II, Ding Y (2005) Increased astrocyte proliferation in rats after running exercise. Neurosci Lett 386(3):160–164
Saur L, de Senna PN, Paim MF, Nascimento Pd, Ilha J, Bagatini PB, Achaval M, Xavier LL (2014) Physical exercise increases GFAP expression and induces morphological changes in hippocampal astrocytes. Brain Struct Funct 219(1):293–302
Alese OO, Mabandla MV (2019) Upregulation of hippocampal synaptophysin, GFAP and mGluR3 in a pilocarpine rat model of epilepsy with history of prolonged febrile seizure. J Chem Neuroanat 100:101659
Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119(1):7–35
Li K, Li J, Zheng J, Qin S (2019) Reactive astrocytes in neurodegenerative diseases. Aging Dis 10(3):664–675
King A, Szekely B, Calapkulu E, Ali H, Rios F, Jones S, Troakes C (2020) The increased densities, but different distributions, of both C3 and S100A10 immunopositive astrocyte-like cells in Alzheimer’s disease brains suggest possible roles for both A1 and A2 astrocytes in the disease pathogenesis. Brain Sci 10(8):503
Hayatdavoudi P, Hosseini M, Hajali V, Hosseini A, Rajabian A (2022) The role of astrocytes in epileptic disorders. Physiol Rep 10(6):1–13
Aronica E, Ravizza T, Zurolo E, Vezzani A (2012) Astrocyte immune responses in epilepsy. Glia 60(8):1258–1268
Maugeri G, D’Agata V, Magrì B, Roggio F, Castorina A, Ravalli S, Di Rosa M, Musumeci G (2021) Neuroprotective effects of physical activity via the adaptation of astrocytes. Cells 10(6):1–13
Fahimi A, Baktir MA, Moghadam S, Mojabi FS, Sumanth K, McNerney M, Ponnusamy R, Salehi A (2017) Physical exercise induces structural alterations in the hippocampal astrocytes: exploring the role of BDNF-TrkB signaling. Brain Struct Funct 222(4):1797–1808
Author information
Authors and Affiliations
Contributions
The authors' contributions are as follows: Conception and design of research: Fariba Karimzadeh, Mansoureh Soleimani. Perform experiments: Saad Bavi, Azam Navazesh, Fahime Zavvari. Analysis and interpretation of data: Homa Rasoolijazi. Prepare the manuscript: Fahime Zavvari. Edit and revise of the manuscript: Fariba Karimzadeh, Mansoureh Soleimani. All authors reads and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflict of interest in this research. There is not any relationship with industry.
Ethical approval
All the experiments were carried out according to the protocol approved by the animal ethics of the Iran University of Medical Sciences, Tehran, Iran (IR.IUMS.RFC.1398.1287).
Informed consent
For this type of study formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bavi, S., Navazesh, A., Rasoolijazi, H. et al. Modulatory effect of exercise on reactive astrocytes in the somatosensory cortex of epileptic rats. Sport Sci Health 20, 65–72 (2024). https://doi.org/10.1007/s11332-023-01065-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11332-023-01065-9