Abstract
Purpose
The purpose of this preliminary study was to compare the effects of 1-h sitting with and without short active breaks on muscle stiffness as measured by shear-wave elastography (SWE).
Methods
The participants (7 females, 3 males; age: 24.9 ± 1.2 years) completed two (with and without active breaks) 1-h sitting exposures on separate days. Active breaks (2–3 min) were performed at 20 min and 40 min time marks and comprised simple stretching and activation exercises. Before, during (30 min) and after (1 h) of sitting, shear modulus of upper trapezius, lumbar region of erector spinae and rectus femoris muscles was measured with SWE.
Results
Statistically significant effects of sitting exposure in erector spinae muscle stiffness were noted (p = 0.041; η2 = 0.38). There were no other statistically significant effects of sitting exposure or condition (with/without breaks).
Conclusions
Although few statistically significant effects were detected, the trends in this preliminary trial suggest that prolonged sitting increases muscle stiffness and warrants further investigation of short active breaks with larger sample sizes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Office work is characterized by long hours of sitting. Occupational sitting is associated with numerous musculoskeletal disorders, such as low back pain [1, 2] and neck pain [2]. Prolonged sitting increases spinal stiffness [3] and low back discomfort [4]. Working with the computer also affects the neck region. In particular, increased muscle stiffness [5, 6] and increased EMG activity [7] of the upper trapezius (UT) muscle may contribute to the development of neck pain. In recent years, several interventions have been proposed to mitigate the negative effects of sitting, including sit–stand desks and other active workstations, such as cycling and treadmill desks [8], as well as performing active breaks [9]. While sit–stand desks and active workstations have the potential to reduce sitting time [10], increase energy expenditure [11, 12] and reduce discomfort [13], there are some associated drawbacks, such as cost, potential difficulties with integration into office environment, and questionable acceptance by some users [14].
The implementation of active breaks is a promising alternative to active workstations. Indeed, a recent review found that active breaks were effective in reducing discomfort and pain, and did not negatively impact work productivity (Waongenngarm et al., 2018). On the other hand, less is known about the effects of active breaks on muscle stiffness. Both muscle stiffness [15, 16] and spinal stiffness [3] have been shown to be increased during sitting. As the possible underlying mechanisms of increased stiffness include restricted blood flow and decreased muscle tissue oxygenation caused by static muscle contractions [17], active breaks with dynamic exercises could be an optimal approach to mitigate the increase in muscle stiffness observed after prolonged sitting. Recently, it has been shown that regular muscular contractions may ameliorate the increase in stiffness of the erector spinae (ES) muscle [16]; however, the contractions were induced by electrical stimulation. Another study reported a similar effect for an 8-min roller massage intervention [15]. Performing short (i.e., ~ 1–3 min) but frequent (e.g., every 20–30 min) active breaks could be a feasible alternative for workers that cannot afford or are not allow to take longer breaks from work. While short active breaks are known to positively affect physical and mental wellbeing of office workers [18], to the best of our knowledge, no study has yet investigated the effect of active breaks on muscle stiffness during sitting.
Previous studies have measured muscle stiffness during sitting work using myotonometry [15, 16]. Although this method has high reliability, its validity has been questioned [19]. In recent years, ultrasound-based shear wave elastography (SWE) has been increasingly used for assessing muscle stiffness [20]. In this method, a shear wave in the tissue is excited with ultrasonic acoustic waves, whereupon the speed of the wave propagation along the tissue is measured [21]. By multiplying the tissue density and the propagation speed of the shear wave, a shear modulus (representing muscle stiffness) is calculated. The shear modulus of the UT muscle has been linked to neck pain [5], but not in all studies [22]. Moreover, the stiffness of the back muscles (ES and multifidus) has been implicated as a potential factor in LBP [23, 24]. However, it is still unknown whether the shear modulus is increased during sitting work and whether short active breaks have the potential to mitigate the increase in muscle stiffness.
The purpose of this study was to preliminary assess the effect of sitting with and without short active breaks on muscle stiffness as measured by SWE (shear modulus). While previous studies have focused on low back muscles [15, 16], we also included the UT muscle due to its potential role in neck disorders [5]. In addition, we also assessed the rectus femoris (RF) muscle (typically not highly active during sitting [25]) to reveal if muscle stiffness may also increase in inactive muscles. We hypothesized that shear modulus in all muscles would increase after sitting, and that this increase would be neutralized or ameliorated with short active breaks.
Methods
Participants
A convenience sample of 10 (7 females, 3 males) young healthy students of Faculty of Health Sciences was recruited for the study (age 24.9 ± 1.2 years; body height = 171.7 ± 10.2 cm, body mass 66.7 ± 13.2 kg). The sample size was calculated using G*Power software, version 3.1.9.2 (Kiel, Germany). Power analysis demonstrated that the sample size needed for the present study were 8 subjects, with a moderate effect size of 0.50, an α error probability of 0.05, and a statistical power of 0.80. The effect size was estimated from previous studies assessing muscle stiffness with SWE during sitting [15, 16]. The inclusion criteria were age > 18 years. The participants who reported musculoskeletal injuries in the last 12 months or having any non-communicable chronic diseases or neuromuscular problems were excluded from the study. Before the measurements, each participant was thoroughly informed about the aims and procedures of the study. The participants were requested to sign an informed consent form prior to participation. The study was approved by the National Medical Ethics Committee of Slovenia (approval number 0120–690/2017/8) and was conducted in accordance with the Declaration of Helsinki.
Study design and experimental conditions
This was a randomized cross-over study, wherein the participants came to the laboratory for two experimental sessions. They were exposed to 1 h of sitting (sitting condition) in one session and to 1 h of sitting with active breaks in the second session. The order of the conditions was counterbalanced across participants, and the two experimental sessions were performed 7–10 days apart, at the same time of the day. The participants were asked to refrain from any vigorous physical activity 48 h before each session. Upon arriving to the laboratory, the participants rested for 10 min in relaxed prone position to dissipate any effect of previous activity on muscle shear modulus. Then, baseline measurements were conducted for erector spinae muscles (ES), with the participants remaining in prone position. Then, the shear modulus was also assessed for upper trapezius (UT) and rectus femoris (RF) muscles, with the participants switching to sitting posture. All measurements were performed only on the right side of the body.
Immediately after UT and RF assessments, the participants were exposed to 1 h of sitting. The participants were asked to bring their own laptops and perform their ongoing work on the computer. They were seated in an office chair (Ergoles Enjoy, Ergoles Ltd., Ljubljana, Slovenia) with back support and hand rests. The seat height was adjusted so that knee and hip angles were ~ 90°. In sitting alone condition, the participants performed their work undisturbed for 60 min, with a very short break at 30 min to measure UT and RF stiffness again. These two muscles could be measured without a need to change the sitting position. At 60 min, UT and RF were measured first, after which the participants switched to lying prone position for the final measurement of ES. In active break condition, two very short (~ 2 to 3 min) breaks (details below) were performed at 20- and 40-min timestamps. Otherwise, the order of the procedures was exactly the same as in the sitting alone condition. Desk height and all chair settings were kept identical in both sessions.
Assessment of shear modulus
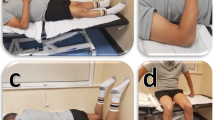
Before the measurements, the locations for the placement of the ultrasound probe were determined and marked with a semi-permanent ink. The probe was always positioned in parallel with muscle fibers orientation. Shear modulus for ES was assessed in relaxed prone position (Fig. 1A). The ultrasound probe was placed at the level of L1 vertebrae (which was determined through palpation), 3 cm from the midline. For UT and RF shear modulus assessment, the participants were seated in the same chair use for exposure to sitting. They were instructed to place their hands on the hand rests (to prevent UT activity) and to relax completely while leaning into the backrest (to prevent RF activity which would arise in case of anterior tilting of the trunk and pelvis). For the RF, the location was set at 50% of the distance between spina iliaca superior anterior and the superior aspect of the patella [26] (Fig. 1B). The probe location for the UT was 50% of the distance between the acromion and the C7 vertebra [27] (Fig. 1C).
We used the Resona 7 ultrasound device (Mindray, Shenzhen, China), set to musculoskeletal mode. The muscle tissue density was assumed to be 1000 kg/m3. A 4-cm linear ultrasound probe (Model L11-3U, Mindray, Shenzhen, China), with a generous amount of water-soluble hypoallergenic ultrasound gel (AquaUltra Basic, Ultragel, Budapest, Hungary) was used. The region of interest was fixed at 1.5 × 1.5 cm for ES, 1.0 × 1.0 cm for RF, and 0.5 × 1.0 cm for the UT. This was done to ensure that only the properties of the muscle tissue were captured. The depth of the region of interest was determined individually for each participant and was kept constant across sessions. As recommended, we ensured that the depth of the region of interest was never more than 4 cm, as the reliability of shear modulus assessment decreases with tissue depth [28]. The outcome measure for each trial was the mean value of eight quick consecutive scans, which is the maximum storage capacity of the device. The device displays the variability of the measurements (standard deviation expressed as % of the mean value). If the variability of the values within the 8 scans was above 15%, the trial was repeated. Three trials were collected at each time point (total 24 scans for UT and RF, and 16 for ES), and the mean value of all scans was taken for further analysis. The device interface displays the mean shear modulus value on the screen, which was transcribed into pre-prepared sheets on a personal computer. In addition, the interface displays scan-by-scan variability, which enabled us to quickly perform an additional trial if the variability exceeded 15%. Figure 2 shows examples of ultrasound scans with regions of interests for ES (Fig. 2A) and UT (Fig. 2B). Previous studies in our laboratory, using the same device and very similar procedures, reported high intra- and inter-session reliability for UT and biceps femoris muscles [29, 30].
Active breaks
Active breaks were designed to be easy to do without a need for extra equipment, and to be feasible to perform in a short period of time (~ 3 min). Moreover, versatile exercises were chosen to reflect the real-life situations. Specifically, the active breaks included the following exercises: (a) upper trapezius stretch; (b) hip flexors stretch; (c) front plank with support on the table; (d) latissimus dorsi stretch; (e) 10 squats; (f) briefly walking around the room and performing shoulder rotations. Each exercise was performed for ~ 15 s and repeated twice. The active breaks were supervised by the experiment supervisors, who is an expert in exercise science (i.e., kinesiology graduate).
Statistical analyses
The data were statistically processed in the SPSS 25 software (IBM, New York, USA). Descriptive statistics is presented as mean ± standard deviation. We assessed within-day reliability (using consecutive trials from the first visit) and test–retest reliability (using baseline values from each visit) by calculating intra-class correlation coefficient (ICC; model 2,1), which was interpreted as: fair (0.40–0.59), moderate (0.60–0.74), good (0.75–0.90) or excellent (> 0.90) [31]. The effects of time (i.e., time within the sessions – 0, 30 and 60 min) and condition (sitting alone and with active breaks) were tested with 2-way repeated measures analysis of variance or paired-sample t tests. The level of statistical significance was set at α < 0.05 for all analyses. Given the preliminary nature of this study and the small sample size, we also closely examined the partial eta-squared (η2) to estimate the effect size. The effect sizes were interpreted as small (< 0.06), moderate (0.06–0.14) and large (> 0.14) [32].
Results
Reliability
The data for two participants for RF muscle were excluded as they presented as outliers (values at 33.3–54.1 kPa. This was done because these values are clearly outside the range reported in the previous literature for relaxed RF muscle [26]. Moreover, these values were clear outliers by the statistical standards for detecting outliers [33]. The within-day reliability was excellent for UT (ICC = 0.94) and RF muscles (ICC = 0.93), and good for ES muscle (ICC = 0.88). The test–retest reliability was good for all of the tested muscles (ICC = 0.84 for ES; 0.80 for UT and 0.83 for RF).
Changes in shear modulus after sitting and the effect of active breaks
For the ES muscle, we found a statistically significant main effect of time (p = 0.041; η2 = 0.38), but no statistically significant effect of condition (p = 0.453) or interaction (p = 0.381). Based on the descriptive statistics, shear modulus increased in time (from 15.3 ± 2.7 kPa to 17.1 ± 3.6 kPa in sitting condition; from 15.3 ± 2.4 kPa to 16.0 ± 1.9 kPa in active breaks condition; Fig. 3A. However, the interaction effect was not statistically significant (p > 0.05; η2 = 0.08), which. Across all participants, the maximal value after 60 min of sitting alone was 22.3 kPa and 19.4 kPa after sitting with active breaks.
For the UT muscle, we found no statistically significant main effects nor interactions (p = 0.389–0.948), despite the moderate interaction effect size. (η2 = 0.11). In sitting alone condition, the shear modulus values were relatively constant (pre: 16.4 ± 6.9 kPa; post 30 min 16.7 ± 6.3 kPa; post 60 min 17.1 ± 5.1 kPa). On the other hand, shear modulus tended to decrease during active breaks condition (pre: 18.5 ± 7.3 kPa; post 30 min 16.6 ± 4.4 kPa; post 60 min 15.4 ± 5.4 kPa) (Fig. 3B). For this muscle, a high between-participant variability was present, likely limiting the statistical power.
For the RF muscle, we also did not find any statistically significant main effect or interaction (p = 0.152–0.257) despite large effect sizes were large for all (η2 = 0.25–0.36). Since we had to remove two participants from the RF data, the statistical power for this analysis was further reduced. During sitting alone, the baseline value was 9.7 ± 1.8 kPa, which increased to 11.9. ± 2.9 kPa after 30 min and to 11.5 ± 2.7 kPa after 60 min. In the active break condition, the baseline value was 9.6 ± 2.2 kPa, which increased to 10.2 ± 3.0 kPa after 30 min, but then decreased to 9.4 ± 2.8 kPa (Fig. 3C). Across all participants, the maximal value after 60 min of sitting alone was 15.8 kPa and 12.1 kPa after sitting with active breaks.
Discussion
The purpose of this study was to explore the effect of 1-h sitting on muscle stiffness quantified by shear-wave elastography, and to assess the effect of including short active breaks. While this preliminary investigation is limited by small sample size, the trends indicate that sitting might increase shear modulus across different muscles, and that performing short active breaks during sitting work may mitigate these effects.
Out study demonstrated statistically significant effects of sitting on ES stiffness, but not on RF and UT stiffness, although the trends in the data indicate that increase in stiffness would be confirmed with larger sample. The effects of prolonged sitting on trunk/spine stiffness have been known for a long time [3]. However, the changes on the muscular level have been largely unknown. Indeed, changes in trunk/spine stiffness can have multiple underlying mechanisms, such as increased intrinsic tissue stiffness, baseline muscle activity, and muscle reflex gains [34]. Together with recent studies [15, 16], this study adds to the evidence that prolonged sitting increases muscle stiffness. While further studies are urgently needed to confirm our findings with larger sample sizes, the increases in ES muscle stiffness (+ 11%) were consistent with the results obtained by Kett et al. (2021), who reported even larger (+ 16%) increase after a longer time (4.5 h) of sitting. Note that previous studies have measured muscle stiffness using myotonometry (Kett et al., 2021; Kett and Sichting, 2020), which has its own limitations although it has been recently shown that its outcomes are correlated with shear modulus [35]. The exact mechanisms underlying the changes in muscle stiffness in either muscle are difficult to discern. It has been suggested that static sitting posture negatively impacts muscle metabolism, blood flow, and oxygenation [36], which potentially contributes to the formation of persisting actin–myosin cross-bridge formations [37] and development of trigger points [38], which could increase the shear modulus [39]. Regardless of the exact mechanism, the increased muscle stiffness could contribute to the development of chronic musculoskeletal diseases. Indeed, increased muscle stiffness of UT muscle has already been implicated as a factor in development of neck pain [5, 6].
It is clear that individual, organizational, and environmental interventions are needed to counteract the effect of prolonged workplace sitting [40]. While several studies have investigated the effects of various interventions on workplace sitting time [10, 41], discomfort or pain [9, 13, 42], and energy expenditure [11, 12], fewer studies have involved muscle stiffness assessments. In sport science studies, a decrease in muscle stiffness after cycling and foam-rolling activities has been shown [43]. However, the feasibility to perform such activities during office work is questionable. Apart from the aforementioned studies that confirmed the potential of roller massage [16] and electrical stimulation [15], one recent study explored the effects of prolonged sitting, standing, and an alternating sit–stand on trunk stiffness [44]. While the alternating the two postures was associated with the lowest discomfort, the trunk stiffness was the highest in this condition [44]. Standing alone was not more comfortable than sitting alone, and while research is being done to determine the optimal sit:stand ratio [45] or more generally, work:rest ratio [46], sit–stand stations are not without limitations [14].
Short and frequent active exercise breaks could be a feasible alternative, which was also supported by the results of this studies. Indeed, a recent systematic review reported positive metabolic and mental/cognitive effects of short active breaks in office workers [18]. The mitigation of the increases in muscle stiffness, as observed in this study, could contribute to prevention of musculoskeletal disorders [5, 6] and workplace discomfort [9]. Given that active breaks require the interruption of work, future studies are needed to determine the optimal length and frequency of the breaks to improve the feasibility of the real-life implementation. Although our active breaks are relatively short (~ 2–3 min), the implementation of such breaks might not be always possible.
The main limitation of this study is its preliminary nature. Further research with larger sample sizes will need to be performed to confirm our findings. While our initial power analysis indicated that this small sample should suffice, we only used 1-h sitting exposure (compared to 4.5 h in previous studies), which could result in much smaller effects. Future studies should consider using longer sitting periods to study the effect of active breaks on muscle stiffness. In addition, only a small region of each muscle has been assessed. While this was done to minimize the interruptions of sitting and the time in between sitting exposure and final assessments, region-specific stiffness and its changes within the muscle have been shown with SWE [47]. Investigating region-specific changes could be another interesting avenue for future research. We asked participants to bring their own laptops and perform their ongoing work. While the work they did was generally very similar (typing, interspersed with reading and clicking), and this contributes to ecological validity of the study, the work was not controlled by the examiners, which could contribute to the variability among the participants and between the sessions. Moving the participants out of the chair to take ES measures could also affect the results; although challenging, it should be considered in future to attempt to measure ES while sitting. Finally, muscle shear modulus obtained by SWE is dependent on probe pressure [48]. While extra care was taken to maintain similar probe pressure for all participants and the reliability of the SWE was good to excellent, probe pressure could nevertheless be an additional factor contributing to the variability in the scores.
Conclusions
This study indicates that stiffness in ES muscles is increased during prolonged sitting, and might also increase in UT and RF muscles. Short (~ 2–3 min) active breaks could be effective in mitigating the increase in muscle stiffness; however, statistically significant effects could not be confirmed with this preliminary investigation. This study is the first to investigate muscle stiffness changes with prolong sitting using shear modulus, demonstrating the potential of SWE applied ergonomics research. Future studies with larger sample sizes are needed to investigate the effectiveness of different active breaks on muscle shear modulus through different periods of prolonged sitting.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Lis AM, Black KM, Korn H, Nordin M (2007) Association between sitting and occupational LBP. Eur Spine J 16:283–298. https://doi.org/10.1007/s00586-006-0143-7
Dzakpasu FQS, Carver A, Brakenridge CJ, et al (2021) Musculoskeletal pain and sedentary behaviour in occupational and non-occupational settings: a systematic review with meta-analysis. Int J Behav Nutr Phys Act 18:. https://doi.org/10.1186/s12966-021-01191-y
Beach TAC, Parkinson RJ, Stothart JP, Callaghan JP (2005) Effects of prolonged sitting on the passive flexion stiffness of the in vivo lumbar spine. Spine J 5:145–154. https://doi.org/10.1016/j.spinee.2004.07.036
Waongenngarm P, Rajaratnam BS, Janwantanakul P (2015) Perceived body discomfort and trunk muscle activity in three prolonged sitting postures. J Phys Ther Sci 27:2183–2187. https://doi.org/10.1589/jpts.27.2183
Taş S, Korkusuz F, Erden Z (2018) Neck muscle stiffness in participants with and without chronic neck pain: a shear-wave elastography study. J Manipulative Physiol Ther 41:580–588. https://doi.org/10.1016/j.jmpt.2018.01.007
Kocur P, Wilski M, Lewandowski J, Łochyński D (2019) Female office workers with moderate neck pain have increased anterior positioning of the cervical spine and stiffness of upper trapezius myofascial tissue in sitting posture. PM R 11:476–482. https://doi.org/10.1016/j.pmrj.2018.07.002
Leonard JH, Kok KS, Ayiesha R et al (2010) Prolonged writing task: comparison of electromyographic analysis of upper trapezius muscle in subjects with or without neck pain. Clin Ter 161:29–33
Ojo SO, Bailey DP, Chater AM, Hewson DJ (2018) The impact of active workstations on workplace productivity and performance: a systematic review. Int J Environ Res Public Health 15:. https://doi.org/10.3390/ijerph15030417
Waongenngarm P, Areerak K, Janwantanakul P (2018) The effects of breaks on low back pain, discomfort, and work productivity in office workers: a systematic review of randomized and non-randomized controlled trials. Appl Ergon 68:230–239. https://doi.org/10.1016/j.apergo.2017.12.003
Shrestha N, Kukkonen-Harjula KT, Verbeek JH, et al (2016) Workplace interventions for reducing sitting at work. Cochrane database Syst Rev 3:CD010912. https://doi.org/10.1002/14651858.CD010912.pub3
Tudor-Locke C, Schuna JM, Frensham LJ, Proenca M (2014) Changing the way we work: elevating energy expenditure with workstation alternatives. Int J Obes 38:755–765. https://doi.org/10.1038/ijo.2013.223
Podrekar N, Kozinc Ž, Šarabon N (2021) Effects of cycle and treadmill desks on energy expenditure and cardiometabolic parameters in sedentary workers: review and meta-analysis. Int J Occup Saf Ergon 27:728–736. https://doi.org/10.1080/10803548.2018.1562688
Agarwal S, Steinmaus C, Harris-Adamson C (2018) Sit-stand workstations and impact on low back discomfort: a systematic review and meta-analysis. Ergonomics 61:538–552. https://doi.org/10.1080/00140139.2017.1402960
Hall J, Kay T, McConnell A, Mansfield L (2019) “Why Would You Want To Stand?” an Account of the Lived Experience of Employees Taking Part in a Workplace Sit-Stand Desk Intervention. BMC Public Health 19:. https://doi.org/10.1186/s12889-019-8038-9
Kett AR, Milani TL, Sichting F (2021) Sitting for Too Long, Moving Too Little: Regular Muscle Contractions Can Reduce Muscle Stiffness During Prolonged Periods of Chair-Sitting. Front Sport Act Living 3:. https://doi.org/10.3389/fspor.2021.760533
Kett AR, Sichting F (2020) Sedentary behaviour at work increases muscle stiffness of the back: Why roller massage has potential as an active break intervention. Appl Ergon 82:102947. https://doi.org/10.1016/j.apergo.2019.102947
Visser B, Van Dieën JH (2006) Pathophysiology of upper extremity muscle disorders. J Electromyogr Kinesiol 16:1–16. https://doi.org/10.1016/j.jelekin.2005.06.005
Radwan A, Barnes L, DeResh R, et al (2022) Effects of active microbreaks on the physical and mental well-being of office workers: A systematic review. Cogent Eng 9:. https://doi.org/10.1080/23311916.2022.2026206
Pamukoff DN, Bell SE, Ryan ED, Blackburn JT (2016) The myotonometer: not a valid measurement tool for active hamstring musculotendinous stiffness. J Sport Rehabil 25:111–116
Taljanovic MS, Gimber LH, Becker GW et al (2017) Shear-wave elastography: basic physics and musculoskeletal applications. Radiographics 37:855–870. https://doi.org/10.1148/rg.2017160116
Blank J, Blomquist M, Arant L, et al (2021) Characterizing musculoskeletal tissue mechanics based on shear wave propagation - a 1 systematic review of current methods and reported measurements
Dieterich AV, Yavuz UŞ, Petzke F et al (2020) Neck muscle stiffness measured with shear wave elastography in women with chronic nonspecific neck pain. J Orthop Sports Phys Ther 50:179–188. https://doi.org/10.2519/jospt.2020.8821
Koppenhaver S, Gaffney E, Oates A, et al (2020) Lumbar muscle stiffness is different in individuals with low back pain than asymptomatic controls and is associated with pain and disability, but not common physical examination findings. Musculoskelet Sci Pract 45:. https://doi.org/10.1016/j.msksp.2019.102078
Murillo C, Falla D, Sanderson A et al (2019) Shear wave elastography investigation of multifidus stiffness in individuals with low back pain. J Electromyogr Kinesiol 47:19–24. https://doi.org/10.1016/j.jelekin.2019.05.004
Arborelius UP, Wretenberg P, Lindberg F (1992) The effects of armrests and high seat heights on lower-limb joint load and muscular activity during sitting and rising. Ergonomics 35:1377–1391. https://doi.org/10.1080/00140139208967399
Takeuchi K, Sato S, Kiyono R, et al (2021) High-Intensity Static Stretching in Quadriceps Is Affected More by Its Intensity Than Its Duration. Front Physiol 12:. https://doi.org/10.3389/fphys.2021.709655
Yanase K, Ikezoe T, Nakamura M, et al (2021) Effective muscle elongation positions for the neck extensor muscles: An ultrasonic shear wave elastography study. J Electromyogr Kinesiol 60:. https://doi.org/10.1016/j.jelekin.2021.102569
Alfuraih AM, O’Connor P, Hensor E et al (2018) The effect of unit, depth, and probe load on the reliability of muscle shear wave elastography: Variables affecting reliability of SWE. J Clin Ultrasound 46:108–115. https://doi.org/10.1002/jcu.22534
Kozinc Ž, Šarabon N (2020) Shear-wave elastography for assessment of trapezius muscle stiffness: Reliability and association with low-level muscle activity. PLoS One 15:. https://doi.org/10.1371/journal.pone.0234359
Šarabon N, Kozinc Ž, Podrekar N (2019) Using shear-wave elastography in skeletal muscle: a repeatability and reproducibility study on biceps femoris muscle. PLoS One 14:. https://doi.org/10.1371/journal.pone.0222008
Fleiss JL (1999) The design and analysis of clinical experiments. Wiley, Hoboken, NJ, USA
Olejnik S, Algina J (2003) Generalized eta and omega squared statistics: measures of effect size for some common research designs. Psychol Methods 8:434–447
Leys C, Ley C, Klein O et al (2013) Detecting outliers: Do not use standard deviation around the mean, use absolute deviation around the median. J Exp Soc Psychol 49:764–766. https://doi.org/10.1016/j.jesp.2013.03.013
Voglar M, Wamerdam J, Kingma I, et al (2016) Prolonged intermittent trunk flexion increases trunk muscles reflex gains and trunk stiffness. PLoS One 11:. https://doi.org/10.1371/journal.pone.0162703
Do Y, Lall PS, Lee H (2021) Assessing the effects of aging on muscle stiffness using shear wave elastography and myotonometer. Healthc 9:. https://doi.org/10.3390/healthcare9121733
Kell RT, Bhambhani Y (2008) Relationship between erector spinae muscle oxygenation via in vivo near infrared spectroscopy and static endurance time in healthy males. Eur J Appl Physiol 102:243–250. https://doi.org/10.1007/s00421-007-0577-6
Proske U, Morgan DL (1999) Do cross-bridges contribute to the tension during stretch of passive muscle? J Muscle Res Cell Motil 20:433–442. https://doi.org/10.1023/A:1005573625675
Fischer MJ, Horvath G, Krismer M et al (2018) Evaluation of mitochondrial function in chronic myofascial trigger points—a prospective cohort pilot study using high-resolution respirometry. BMC Musculoskelet Disord 19:388. https://doi.org/10.1186/s12891-018-2307-0
Ertekin E, Kasar ZS, Turkdogan FT (2021) Is early diagnosis of myofascial pain syndrome possible with the detection of latent trigger points by shear wave elastography? Polish J Radiol 86:e461–e466. https://doi.org/10.5114/pjr.2021.108537
Bailey DP (2021) Sedentary behaviour in the workplace: prevalence, health implications and interventions. Br Med Bull 137:42–50. https://doi.org/10.1093/bmb/ldaa039
Peachey MM, Richardson J, V Tang A, et al (2020) Environmental, behavioural and multicomponent interventions to reduce adults’ sitting time: A systematic review and meta-analysis. Br J Sports Med 54:315–325. https://doi.org/10.1136/bjsports-2017-098968
Kar G, Hedge A (2020) Effects of a sit-stand-walk intervention on musculoskeletal discomfort, productivity, and perceived physical and mental fatigue, for computer-based work. Int J Ind Ergon 78:. https://doi.org/10.1016/j.ergon.2020.102983
Morales-Artacho AJ, Lacourpaille L, Guilhem G (2017) Effects of warm-up on hamstring muscles stiffness: cycling vs foam rolling. Scand J Med Sci Sport 27:1959–1969. https://doi.org/10.1111/sms.12832
Park J-H, Srinivasan D (2021) The effects of prolonged sitting, standing, and an alternating sit-stand pattern on trunk mechanical stiffness, trunk muscle activation and low back discomfort. Ergonomics 64:983–994. https://doi.org/10.1080/00140139.2021.1886333
Karakolis T, Callaghan JP (2014) The impact of sit-stand office workstations on worker discomfort andproductivity: a review. Appl Ergon 45:799–806. https://doi.org/10.1016/j.apergo.2013.10.001
Chappel SE, Naweed A, Chapman J, et al (2022) Can occupational health professionals successfully apply the Goldilocks Work Paradigm in a simulated work redesign? Ergonomics 1–14. https://doi.org/10.1080/00140139.2022.2067357
Ewertsen C, Carlsen JF, Christiansen IR et al (2016) Evaluation of healthy muscle tissue by strain and shear wave elastography—Dependency on depth and ROI position in relation to underlying bone. Ultrasonics 71:127–133. https://doi.org/10.1016/j.ultras.2016.06.007
Wang X, Hu Y, Zhu J, et al (2020) Effect of acquisition depth and precompression from probe and couplant on shear wave elastography in soft tissue: An in vitro and in vivo study. Quant Imaging Med Surg 10:754–765. https://doi.org/10.21037/QIMS.2020.01.15
Author information
Authors and Affiliations
Contributions
Both authors contributed to the conceptualization of the study, participant recruitment, measurements, data analysis, and manuscript preparation.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose. The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
This study was performed in line with the principles of Declaration of Helsinki. Approval was granted by the National Medical Ethics Committee of Slovenia (approval number 0120–690/2017/8).
Consent to participate
Informed consent was obtained from all individual participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vöröš, P., Kozinc, Ž. Preliminary investigation of the effects of sitting with and without short active breaks on muscle stiffness assessed with shear-wave elastography. Sport Sci Health 19, 1209–1216 (2023). https://doi.org/10.1007/s11332-023-01051-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11332-023-01051-1