Abstract
Aim
In patients with obstructive sleep apnoea (OSA), the benefits of continuous positive airway pressure (CPAP) therapy are increased for every additional hour of daily CPAP usage. However, the data of predictors of extensive usage is scarce, if any. Therefore, we evaluated potential predictors affecting extensive treatment usage.
Methods
In this retrospective study, we compiled an institutional cohort of consecutive patients diagnosed with who started CPAP therapy 1999–2022 and were included in a wireless telemonitoring system in May 2022 (N = 14,394). Patients using CPAP device ≥ 9 h/d were stratified into a younger (< 65 years; N = 124) and an older group (≥ 65 years; N = 131).
Results
We found 255 patients (male 61%) eligible for our study, with a median age of 65 (interquartile range, IQR 55–73) years, and mean body mass index (BMI) of 36 ± 6.9 kg/m2. Median CPAP use was 10 h/d (IQR 10–11). BMI and depressive symptoms (DEPS) in the younger group were higher than in the older group (37.9 ± 7 vs. 34.6 ± 6.4 kg/m2, p < 0.001 and 11 (IQR 5–20) vs. 7 (IQR 5–14), p = 0.01, respectively). During follow-up, the BMI of the younger group increased (39.9 ± 12.5 kg/m2 vs. 37.9 ± 7 kg/m2, p = 0.009). DEPS values decreased in the younger group and became comparable between the groups. In multivariate models, the baseline BMI independently predicted extensive CPAP use among the younger age group, and the mask leak among the older group.
Conclusion
BMI at baseline in the younger and mask leak in the older group could be independent predictive factors for extensive use of CPAP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnoea (OSA) is a common sleep-related breathing disorder. The number of OSA patients is increasing, and the vast majority are still undiagnosed. OSA affects hundreds of millions of individuals worldwide, with a prevalence of 45% considered moderate to severe [1]. Moreover, according to a recent study, the prevalence of clinically diagnosed OSA was 3.7% in the Finnish adult population, and the 1-year incidence was 0.6%2. Multimorbidity was present in 63% of individuals at the time of OSA diagnosis. Of those with incident sleep apnoea, 34% were heavily multimorbid, presenting with four or more comorbidities.
Continuous positive airway pressure (CPAP) is the treatment of choice for moderate to severe OSA [2, 3]. CPAP adherence remains stable in long-term follow-up but does not improve weightloss [4]. To improve OSA symptoms and possible cardiovascular and cerebrovascular outcomes, patients on CPAP therapy should use it more than 4 h per night [5]. Moreover, the clinical significance of the effect of CPAP therapy is improved for every additional hour of CPAP usage per night. However, a clear definition of optimal CPAP adherence is impossible because there are insufficient data on the relationship between usage hours and major clinical outcomes. Previous studies have focused mainly on the predictors of poor adherence to CPAP, or the optimal adherence level needed for the outcome in question [6]. In addition, predictors of extensive CPAP adherence have not been clarified. It may be speculated whether extensive use of CPAP treatment is optimal or possibly linked with health problems or issues with CPAP treatment.
Telemedicine is considered as an approach to evaluate public health challenges in chronic diseases, offering potential cost-effective management options in the long run [7]. Wireless telemonitoring represents an established option to support the routine clinical follow-up of OSA patients receiving CPAP treatment without reducing treatment efficacy or patient satisfaction [8]. This technology enables the evaluation of treatment pressures, air leaks, apnoea-hypopnoea index (AHI), and usage time and the transfer of this data to the patient’s health-care provider on a daily basis [9]. Based on our clinical experience, there is a subgroup of patients using CPAP even more than 12 h a day, but no data are available about the profile of these patients.
This retrospective observational study aims to identify potential predictors of extensive CPAP usage in OSA patients using wireless telemonitoring.
Study design and subject selection
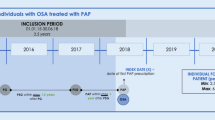
In this retrospective study, we compiled an institutional cohort of consecutive patients (age ≥ 18 years) diagnosed with OSA and who had started CPAP therapy (including fixed PAP and automatic PAP) between 1999 and 2022. Patients were diagnosed with cardiorespiratory polygraphies mainly done at home. These sleep studies were scored according to the rules of the American Academy of Sleep Medicine being valid at the time of performed sleep studies. The telemonitoring (ResTraxx Online System™ or Airview™, ResMed, Sydney, Australia) included also patients who started CPAP treatment during 1999–2012, and were connected to the telemonitoring system in 2013. As this retrospective study included anonymized register data, no ethics committee approval was required according to Finnish legislation. Patients were eligible for our analysis if their average daily CPAP use was ≥ 9 h/day, and they were included in the wireless telemonitoring system (Airview™, ResMed, Sydney, Australia) in May 2022 (N = 14,394).
Data collection
Patients were identified based on the device number. Patients’ demographic data was extracted from Auria Clinical Informatics from Turku University Hospital. This data included age, gender, body mass index (BMI), the severity of sleep apnoea in a diagnostic cardiorespiratory polygraphy, comorbidities at diagnosis, and mental, behavioural, and neurodevelopmental disorders. The scores from subjective sleepiness measured by the Epworth Sleepiness Scale (ESS) [10] (10 points or more is interpreted as excessive daytime sleepiness), psychological distress measured by the General Health Questionnaire 12 (GHQ-12) [11] (3 points or more, the test is “positive” for psychological distress), insomnia symptoms with insomnia severity index (ISI) [12] (15 points or more is interpreted as positive for insomnia symptoms) and depressive symptoms screened by the DEPS scale [13] were included in the collected data. DEPS is a standard self-rating depression questionnaire commonly used in Finland for > 15 years and consists of 10 items that cover the core of depression symptoms. The score ranges between 0 and 30, and scores ≥ 9 suggest depression [13]. Furthermore, CPAP registry data (CPAP use, pressure setting, residual AHI and mask leakage) were obtained from the telemonitoring system. We used the extracted clinical information to evaluate the relationship between extensive CPAP usage and multimorbidity. Multimorbidity was defined as two or more chronic diseases patients had during data collection. The chronic diseases included in this study were hypertension, diabetes mellitus, chronic obstructive pulmonary disease (COPD), morbid obesity with alveolar hypoventilation, ischemic heart diseases, interstitial pulmonary diseases, depression, and any cancer.
Telemonitoring data of all included patients were re-evaluated during the last six months. The patients were divided into two groups for descriptive purposes. Since retirement has an impact on different risk factors for chronic diseases [14, 15], the included patients were stratified according to statutory retirement age in Finland into two groups: before retirement (< 65 years group) and at retirement and after (≥ 65 years group) [16].
Statistical analysis
A Shapiro-Wilk W-test was used to test the distribution of the data. A two-sample t-test or nonparametric Mann-Whitney test was used to compare the continuous variables and variables expressed as mean ± standard deviation (SD) or median and interquartile range (IQR). Categorized variables were analyzed using a chi-square test and expressed as numbers and percentages. The changes in continuous variables during follow-up were tested using paired t-test or Wilcoxon signed ranked test. Correlations are performed to analyze the relations between the variables using the Spearman correlation coefficient. Multiple variables regression was used to determine the effect of CPAP treatment. P values < 0.05 were considered statistically significant. Statistical analyses were done using SPSS v.29.01 (IBM Corporation, New York, USA).
Results
With Insight software™ (ResMed, Sydney, Australia,) the final number was 255 patients eligible for our study (1,9% of those using CPAP), after excluding the patients with missing data in one or more items described above. Of the eligible patients, 124 were younger than 65 years and 131 were 65 years or older. Median age was 65 (IQR 55–73) years, 61% were male, mean BMI 36 ± 6.8 kg/m2, and median of average daily CPAP usage was 10 (IQR 10–11) hours/day. There were no significant differences between the two age groups regarding demographic and clinical variables, except that obesity (BMI) was higher in the younger group, and hypertension was more common in the older group (Table 1).
At the baseline, there were no statistical differences among the groups in terms of subjective sleepiness (ESS), sleep apnoea severity (AHI), psychological distress (GHQ-12) or insomnia symptoms (ISI). However, the baseline BMI and depressive symptoms (DEPS) in the younger group were significantly higher than in the older group (37.9 ± 7 kg/m2 vs. 34.6 ± 6.4 kg/m2, p < 0.001 and 11, (IQR 5–20) vs. 7, (IQR 5–14), p = 0.01, respectively). Compared with baseline, after CPAP treatment, significant decreases in AHI and ESS scores were observed for both groups, while DEPS and ISI scores were significantly changed only in the younger group. Moreover, after CPAP treatment, the BMI of the younger group increased dramatically from 37.2 ± 7.3 to 39.9 ± 12.5, p = 0.006 (78 patients, 63% of the young population). In addition, it became higher than the BMI of ≥ 65 years group after six months of CPAP treatment (p < 0.001). Conversely, DEPS scores after CPAP treatment were comparable among the two groups (9.2 (IQR 5–20) vs. 6 (IQR 3–10), p = 0.6). (Table 2)
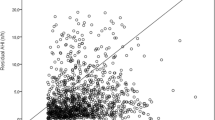
The Spearman correlation test determined the relationship between variables and the extensive CPAP usage. There was a significant correlation between average extensive CPAP use and BMI values that were measured at follow-up in the younger group (r = 0.047, p < 0.001); increasing BMI correlated with higher CPAP usage hours (Fig. 1). Furthermore, there was a significant correlation between the average extensive CPAP usage and differences in BMI (delta) at baseline and follow-up (r = 0.26, p = 0.02) (Fig. 2). However, no correlations existed between the average extensive CPAP usage and other CPAP treatment variables. There was a significant correlation between average extensive CPAP usage and mask leak in the older group (r = 0.2, p = 0.01) (Fig. 3). Conversely, the older group had no correlations between CPAP usage and treatment variables.
In univariable regressions for the younger group, only baseline BMI (β = 0.261; 95% confidence interval [CI] 0.12, 0.16]; P = 0.004) and AHI (β = 0.01; 95% CI [-0.1,0.04)]; P = 0.004) predicted extensive CPAP usage. In the older group, the univariable analysis showed that baseline BMI (β = 0.061; 95% CI [-0.02, 0.045)]; P = 0.001), AHI (β = 0.089; 95% CI [0, 0.015)]; P = 0.01), and mask leak (β = 0.192; 95% CI [0,0.34)]; P = 0.03), could predict the extensive CPAP usage. However, in multiple regression analysis, baseline BMI (β = 0.277; 95% CI [0.14,0.65)]; P = 0.003) and mask leak (β = 0.199; 95% CI [0, 0.37)]; P = 0.04) were only independent predictors for average daily usage of CPAP in the younger and the older group, respectively. Results of the univariable and multiple regressions are shown in Table 3.
Discussion
This is the first study to assess predictors of extensive CPAP usage in patients using wireless telemonitoring. To our knowledge, no previous publications are focusing on the “heavy users” in particular, i.e. patients using CPAP therapy 9 h/day or more on average. The main findings of our study were that although CPAP usage hours were similar in both age groups, the BMI was higher among the younger group than the older group at the beginning of the treatment and augmented over the course of treatment. Moreover, the depression scores were higher at the beginning and improved significantly after the treatment in the younger group, whilst in the older group, there was no significant improvement after the treatment. The BMI and mask leak were independent predictors of extensive CPAP usage in the younger and older groups, respectively.
BMI and extensive CPAP usage
Obesity is one of the critical factors for OSA, and many studies have consistently found an association between gain of weight and the risk of OSA [17]. The effects of CPAP on BMI and weight are unclear. However, some studies have linked CPAP therapy to reduced resting metabolic rate due to the hormonal impacts possible resulting the weight gain [17]. Previous studies assessed the effect of CPAP on body weight in OSA patients. Quan et al. evaluated the impacts of CPAP treatment in OSA patients for six months in a randomized controlled multicentre trial. They demonstrated that CPAP treatment was associated with weight gain [18]. The weight change was associated with CPAP adherence with each hour per night of CPAP usage associated with a mean 0.42 kg increase in weight over six months. However, the study could not observe any associations between the CPAP treatment and related factors such as age, gender, race, and baseline BMI.
Furthermore, Redenius et al. [19], in a retrospective study of 183 OSA patients treated with CPAP extended to 14 months, showed that women were more likely to gain weight after CPAP usage. Our study has a considerably larger and more extended follow-up period and, therefore is more likely to detect precisely the factors that impact CPAP use. Our findings are statistically consistent with those of Myllylä et al. [4], who evaluated OSA patients who used CPAP treatment for more than six years in a big cohort study (1000 patients), and the main finding was that weight gainers after CPAP treatment were significantly younger and had severe obesity at the beginning of the treatment. Inflammatory cytokines, tumor necrosis factor-α (TNFα) and interleukin-6 (IL-6), both of which produce sleepiness and fatigue, are elevated in OSA and obesity, which might lead to extensive usage of CPAP therapy [20]. In addition to hypercytokinemia, younger patients are likely to have higher work load and other coincident demands of daily life compared to older patients, which might explain the different effect of obesity between the age groups.
Daytime sleepiness and CPAP
OSA patients suffer excessive daytime sleepiness, which can negatively affect daily functioning, cognition, mood, and other aspects of well-being [21]. The Epworth Sleepiness Scale (ESS) is often used to assess daytime sleepiness, which is considered to be one of the most common symptoms related to OSA [22]. Successful CPAP treatment for OSA in adherent patients often leads to symptom relief within a few weeks [23]. However, sleepiness persists in 5–9% of OSA patients despite CPAP treatment [5, 24, 25]. Furthermore, ESADA, a prospective multicentre study involving over 30,000 suspected OSA patients to explore predictors of persistent residual excessive daytime sleepiness among many factors, concluded that hours of CPAP use and length of follow-up are the significant predictive factors of residual excessive daytime sleepiness [26]. Our study showed a beneficial effect of CPAP therapy on daytime sleepiness in the long term, and significant decreases in ESS scores were observed in all groups. Our results are in line with the Avlonitou et al. study assessing the quality of life after six months for 50 OSA patients using CPAP treatment. They demonstrated that OSA patients who adhere to night-time CPAP therapy significantly improve their daytime sleepiness [27].
Depression and CPAP
Doctor diagnosed depression was highly prevalent (30%) in our cohort of extensive CPAP users, and nearly threefold compared to that of the Finnish nationwide cohort of OSA patients [28]. Our study showed that the younger group’s DEPS score significantly declined after extensive CPAP treatment compared with their baseline score. However, the DEPS score in the older group was unremarkable at baseline and did not change after the CPAP treatment. DEPS score is an established depression questionnaire in Finland for 25 years and is recommended to identify the severity of depression in primary care patients [29]. Previous study [30] evaluated depression scores at follow-up after CPAP treatment in the group at an age comparable to the age of the younger group of our study (55 years). It showed the improvement in the depression scores was dependent on CPAP adherence. The connection between CPAP treatment and the improvement of depression symptoms is uncertain. Nevertheless, previous studies have identified that the reversal in various pathophysiological mechanisms, such as apnoea-induced sleep fragmentation, excessive daytime sleepiness, and decreased grey matter due to hypoxemia, were associated with CPAP treatment [30]. In contrast, a previous multicentre randomized controlled trial evaluated the effect of CPAP treatment in elderly patients (over 70 years old) with moderate OSA regarding quality of life. It showed there was no effect of CPAP treatment on the degree of depression or anxiety [31]. Our results extend with these observations by showing that CPAP use has a significant impact on the depression of the younger group but not the older group.
Mask leak and extensive CPAP usage
In our study, there was a weak correlation between extensive CPAP usage and mask leak in the older group, and mask leak was considered an independent predictor for extensive CPAP use in the older group. A previous large retrospective study evaluated the impact of age on CPAP use and suggested that the increase of the mask leak with CPAP use maybe explained by increased CPAP use with increasing age [32]. However, in our results, the mask leak was very low, and there was no significant difference between the younger and older groups. Furthermore, previous study evaluated the predictors of CPAP compliance in OSA patients with metabolic syndrome for eight weeks and showed that high mask leak was the only independent predictor of CPAP compliance [33].
Our study has some limitations. First, it was a unicentric retrospective study conducted on a moderate number of Caucasian patients. Secondly, even though we have identified a potentially large patient population, only a small number of patients were finally included in this study. Due to missing values, we did not evaluate the waist circumference for all patients or include it in this study. Furthermore, the sample size of patients with cardiovascular disease was small and lacked the statistical power to assess the impact of extensive CPAP use on cardiovascular disease. Moreover, we did not consider other factors, such as the type of mask (oronasal mask/ nasal mask) or CPAP devices or sedative hypnotics, that may influence the predictive value of CPAP usage. Although we evaluated the predictiveness of extensive CPAP usage by dividing the group study according to the pension age, we did not assess the socioeconomic and personality factors that could affect CPAP usage, like employment status and education, especially among the younger group [34]. Our study did not evaluate the adverse effects of extensive CPAP usage. Not all included patients were evaluated by ISI score because it has only recently used in our centre. Finally, we did not have a matched group of patients using CPAP less than 9 h, and therefore cannot compare the predictors of CPAP usage over the entire range usage hours.
Conclusion
In our practice, extensive CPAP usage is associated with weight gain in patients with OSA less than 65 years old already during the first months of CPAP treatment. Furthermore, our data indicate that BMI among the younger patients and mask leak in the older ones could be predictive factors for extensive usage of CPAP. Further prospective multicentre studies are recommended to evaluate predictive factors for extensive usage of CPAP.
Data availability
This manuscript has no associated data.
References
Benjafield AV, Ayas NT, Eastwood PR et al (2019) Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med 7(8):687–698. https://doi.org/10.1016/S2213-2600(19)30198-5
Gay P, Weaver T, Loube D, Iber C (2006) Evaluation of Positive Airway Pressure Treatment for Sleep Related Breathing Disorders in adults. Sleep 29(3):381–401. https://doi.org/10.1093/sleep/29.3.381
Cao MT, Sternbach JM, Guilleminault C (2017) Continuous positive airway pressure therapy in obstuctive sleep apnea: benefits and alternatives. Expert Rev Respir Med 11(4):259–272. https://doi.org/10.1080/17476348.2017.1305893
Myllylä M, Kurki S, Anttalainen U, Saaresranta T, Laitinen T (2016) High adherence to CPAP Treatment does not prevent the continuation of Weight Gain among severely obese OSAS patients. J Clin Sleep Med 12(04):519–528. https://doi.org/10.5664/jcsm.5680
Sánchez-de-la-Torre M, Gracia-Lavedan E, Benitez ID et al (2023) Adherence to CPAP Treatment and the risk of Recurrent Cardiovascular events: a Meta-analysis. JAMA 330(13):1255–1265. https://doi.org/10.1001/jama.2023.17465PMID: 37787793; PMCID: PMC10548300
Bakker JP, Weaver TE, Parthasarathy S et al (2019) Adherence to CPAP: what should we be Aiming for, and how can we get there? Chest 155(6):1272–1287. https://doi.org/10.1016/j.chest.2019.01.012Epub 2019 Jan 23. PMID: 30684472
Aalaei S, Amini M, Mazaheri Habibi MR et al (2022) A telemonitoring system to support CPAP therapy in patients with obstructive sleep apnea: a participatory approach in analysis, design, and evaluation. BMC Med Inf Decis Mak 22(1):168. https://doi.org/10.1186/s12911-022-01912-8PMID: 35754055; PMCID: PMC9235202
Anttalainen U, Melkko S, Hakko S, Laitinen T, Saaresranta T (2016) Telemonitoring of CPAP therapy may save nursing time. Sleep Breath 20(4):1209–1215. https://doi.org/10.1007/s11325-016-1337-9
Fox N, Hirsch-Allen A, Goodfellow E et al (2012) The impact of a Telemedicine Monitoring System on positive Airway pressure adherence in patients with obstructive sleep apnea: a Randomized Controlled Trial. Sleep 35(4):477–481. https://doi.org/10.5665/sleep.1728
Johns MW (1991) A New Method for Measuring Daytime Sleepiness: the Epworth Sleepiness Scale. Sleep 14(6):540–545. https://doi.org/10.1093/sleep/14.6.540
GOLDBERG DP, GATER R, SARTORIUS N et al (1997) The validity of two versions of the GHQ in the WHO study of mental illness in general health care. Psychol Med 27(1):191–197. https://doi.org/10.1017/S0033291796004242
Bastien C (2001) Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med 2(4):297–307. https://doi.org/10.1016/S1389-9457(00)00065-4
Salokangas RKR, Poutanen O, Stengård E (1995) Screening for depression in primary care development and validation of the Depression Scale, a screening instrument for depression. Acta Psychiatr Scand 92(1):10–16. https://doi.org/10.1111/j.1600-0447.1995.tb09536.x
Yu W, Yang Y, Liu X et al (2023) Heterogeneous effects of retirement on the biomedical risk factors for cardiovascular and metabolic diseases: new evidence based on the physical examination database in Shanghai, China. SSM - Popul Heal 21:101333. https://doi.org/10.1016/j.ssmph.2022.101333
Pedron S, Maier W, Peters A et al (2020) The effect of retirement on biomedical and behavioral risk factors for cardiovascular and metabolic disease. Econ Hum Biol 38:100893. https://doi.org/10.1016/j.ehb.2020.100893
Nivalainen S (2023) Retirement intentions and increase in Statutory Retirement Age: 2017 pension reform and intended Retirement Age in Finland. J Aging Soc Policy Published Online April 1:1–22. https://doi.org/10.1080/08959420.2023.2195787
Drager LF, Brunoni AR, Jenner R et al (2015) Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax 70(3):258–264. https://doi.org/10.1136/thoraxjnl-2014-205361Epub 2014 Nov 28. PMID: 25432944
Quan SF, Budhiraja R, Clarke DP et al (2013) Impact of treatment with continuous positive Airway pressure (CPAP) on Weight in Obstructive Sleep Apnea. J Clin Sleep Med 09(10):989–993. https://doi.org/10.5664/jcsm.3064
Redenius R, Murphy C, O’Neill E, Al-Hamwi M, Zallek SN (2008) Does CPAP lead to change in BMI? J Clin Sleep Med 4(3):205–209. http://www.ncbi.nlm.nih.gov/pubmed/18595431
Vgontzas AN, Papanicolaou DA, Bixler EO et al (2000) Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab 85(3):1151–8. https://doi.org/10.1210/jcem.85.3.6484. PMID: 10720054
Lal C, Weaver TE, Bae CJ, Strohl KP (2021) Excessive daytime sleepiness in Obstructive Sleep Apnea. Mechanisms and clinical management. Ann Am Thorac Soc 18(5):757–768. https://doi.org/10.1513/AnnalsATS.202006-696FR
Sunwoo BY, Kaufmann CN, Murez A et al (2023) The language of sleepiness in obstructive sleep apnea beyond the Epworth. Sleep Breath 27(3):1057–1065. https://doi.org/10.1007/s11325-022-02703-1
Pattipati M, Gudavalli G, Zin M et al (2022) Continuous Positive Airway Pressure vs Mandibular Advancement Devices in the treatment of obstructive sleep apnea: an updated systematic review and Meta-analysis. Cureus Published Online January 31. https://doi.org/10.7759/cureus.21759
Gasa M, Tamisier R, Launois SH et al (2013) Residual sleepiness in sleep apnea patients treated by continuous positive airway pressure. J Sleep Res 22(4):389–397. https://doi.org/10.1111/jsr.12039
Guilleminault C, Philip P (1996) Tiredness and somnolence despite initial treatment of obstructive sleep apnea syndrome (what to do when an OSAS patient stays Hypersomnolent despite Treatment). Sleep 19(suppl9):S117–S122. https://doi.org/10.1093/sleep/19.suppl_9.S117
Bonsignore MR, Pepin JL, Cibella F et al (2021) Excessive daytime sleepiness in obstructive sleep apnea patients treated with continuous positive Airway pressure: data from the European Sleep Apnea Database. Front Neurol 12. https://doi.org/10.3389/fneur.2021.690008
Avlonitou E, Kapsimalis F, Varouchakis G, Vardavas CI, Behrakis P (2012) Adherence to CPAP therapy improves quality of life and reduces symptoms among obstructive sleep apnea syndrome patients. Sleep Breath 16(2):563–569. https://doi.org/10.1007/s11325-011-0543-8
Palomäki M, Saaresranta T, Anttalainen U, Partinen M, Keto J, Linna M (2022) Multimorbidity and overall comorbidity of sleep apnoea: a Finnish nationwide study. ERJ Open Res 8(2):00646–02021. https://doi.org/10.1183/23120541.00646-2021
Poutanen O, Koivisto A-M, Kaaria S, Salokangas RKR (2010) The validity of the Depression Scale (DEPS) to assess the severity of depression in primary care patients. Fam Pract 27(5):527–534. https://doi.org/10.1093/fampra/cmq040
Lundetræ RS, Saxvig IW, Lehmann S, Bjorvatn B (2021) Effect of continuous positive airway pressure on symptoms of anxiety and depression in patients with obstructive sleep apnea. Sleep Breath 25(3):1277–1283. https://doi.org/10.1007/s11325-020-02234-7
Ponce S, Pastor E, Orosa B et al (2019) The role of CPAP treatment in elderly patients with moderate obstructive sleep apnoea: a multicentre randomised controlled trial. Eur Respir J 54(2):1900518. https://doi.org/10.1183/13993003.00518-2019
Woehrle H, Graml A, Weinreich G (2011) Age- and gender-dependent adherence with continuous positive airway pressure therapy. Sleep Med 12(10):1034–1036. https://doi.org/10.1016/j.sleep.2011.05.008
Sopkova Z, Dorkova Z, Tkacova R (2009) Predictors of compliance with continuous positive airway pressure treatment in patients with obstructive sleep apnea and metabolic syndrome. Wien Klin Wochenschr 121(11–12):398–404. https://doi.org/10.1007/s00508-009-1181-z
Gulati A, Ali M, Davies M, Quinnell T, Smith I (2017) A prospective observational study to evaluate the effect of social and personality factors on continuous positive airway pressure (CPAP) compliance in obstructive sleep apnoea syndrome. BMC Pulm Med 17(1):56. https://doi.org/10.1186/s12890-017-0393-7
Funding
Turun Yliopistollisen Keskussairaalan Koulutus- ja Tutkimussäätiö (Turku University Central Hospital Education and Research Foundation) provided financial support in the form of individual grant for Fatma Doghman. The sponsor had no role in the design or conduct of this research.
Open Access funding provided by University of Turku (including Turku University Central Hospital).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
As this retrospective study included anonymized register data, no ethics committee approval was required.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Doghman, F., Ballo, H., Anttalainen, U. et al. Factors predictive of extensive use of CPAP treatment in obstructive sleep apnoea. Sleep Breath (2024). https://doi.org/10.1007/s11325-024-03146-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11325-024-03146-6