Abstract
Purpose
This study aimed to conduct a meta-analysis and systematic review of drug-induced sleep endoscopy (DISE) in pediatric conventional obstructive sleep apnea–hypopnea syndrome (OSAHS) without previous upper airway surgery, or comorbidity, to evaluate the change in treatment strategies and to identify obstructive sites observed during DISE. This study aimed to explore the role of DISE in the management of pediatric conventional OSAHS.
Methods
A comprehensive search was conducted using both computerized and manual methods to retrieve relevant case studies on DISE-guided treatment of pediatric conventional OSAHS from databases including PubMed, EMBASE, Cochrane Library, Web of Science, CNKI, WF, and VIP database. The search period extended from database inception to January 2023. Strict inclusion and exclusion criteria were applied to select relevant literature, and data extraction was performed accordingly. Meta-analysis was conducted using the Stata 16.0 software.
Results
A total of 761 patients from four studies were included in the meta-analysis. All pediatric patients had no history of upper airway surgery, craniofacial abnormalities, or syndromes other than OSAHS. The quality assessment revealed that the included studies were of low methodological quality and consisted of non-randomized case studies. Meta-analysis results indicated that in pediatric patients with OSAHS, the obstruction rates observed during DISE were as follows: nasopharyngeal (adenoid) obstruction 93%, soft palate obstruction 35%, oropharyngeal (tonsil) obstruction 76%, tongue base obstruction 32%, supraglottic obstruction 31%, and multi-level obstruction 60%. DISE led to a change in the conventional surgical approach in 45% (95% CI: 29–60%) of patients with OSAHS, providing individualized treatment plans. Postoperative symptoms and sleep-related parameters improved significantly compared to preoperative values, with DISE findings possibly enhancing surgical success rates and potentially avoiding unnecessary procedures.
Conclusion
In some cases, DISE may potentially lead to alterations in conventional surgical approaches for children with OSAHS who had no history of upper airway surgery, craniofacial abnormalities, or other syndromes.. The results of our meta-analysis were in favor of DISE-directed approach for pediatric conventional OSAHS. However, further high-quality randomized controlled trials (RCTs) are warranted in future research to investigate the role of DISE in the management of pediatric OSAHS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pediatric obstructive sleep apnea–hypopnea syndrome (OSAHS) is a clinical condition characterized by recurrent partial or complete upper airway obstruction leading to hypoventilation and respiratory pauses during sleep in children. It is relatively common among children, with a prevalence ranging from 1.2 to 5.7% [1, 2]. However, it is generally believed that the prevalence of OSAHS is underestimated [1, 2]. Depending on the severity, OSAHS can have adverse effects on children’s health, including behavioral problems, neurocognitive function, cardiovascular health, endocrine metabolism, and growth and development [1, 3, 4]. Adenotonsillar hypertrophy and tonsillar enlargement are considered the most common causes of pediatric OSAHS in healthy children. Therefore, the American Academy of Pediatrics (AAP) recommends adenotonsillectomy (T&A) as the first-line treatment for pediatric OSAHS [1]. However, research has shown that the occurrence of persistent OSAHS after T&A surgery ranges from 21 to 75% based on postoperative apnea–hypopnea index (AHI) findings [5,6,7,8]. A meta-analysis of 1079 pediatric OSAHS indicated that up to 33.7% of children continue to experience sleep breathing disorders after T&A [9]. Apart from systemic diseases and related syndromes, multi-level upper airway narrowing or obstruction is considered a significant factor in treatment-resistant obstructive sleep apnea [10, 11]. Therefore, preoperative assessment of upper airway obstruction levels is crucial to improving the effectiveness of OSAHS treatment.
Drug-induced sleep endoscopy (DISE) is a reliable new technique for assessing the upper airway, which can identify obstructive sites missed during awake examination and provide targeted and effective treatment plans for children, including non-surgical and surgical interventions [12]. In children, most DISE procedures are performed on cases of persistent OSAHS after T&A and special populations, such as those with syndromes or neuromuscular abnormalities, and consensus guidelines have been developed for these scenarios [11, 13,14,15]. However, there is ongoing debate about whether pediatric OSAHS without previous upper airway surgery or comorbidity are ideal candidates for DISE. In 2015, Galluzzi et al.‘s systematic review suggested that the widespread use of DISE in pediatric patients with OSAHS is not advisable [16]. However, Boudewyns et al. [17] offered a different perspective, stating that DISE aids in decision-making for routine OSAHS surgeries but requires large-sample and RCT studies to prove this conclusion. It is worth noting that Galluzzi et al.’s study included many special populations, which may have influenced the results. In 2017, Gazzaz et al. [18] found that conventional OSAHS without a history of upper airway surgery, craniofacial abnormalities, or syndromes were ideal candidates for DISE. In their study of 558 patients, 35% of children changed their surgical approach based on the findings of DISE. To investigate the impact of DISE on decision-making for conventional OSAHS (no history of upper airway surgery, craniofacial abnormalities, or other syndromes) surgeries, this study conducts a meta-analysis and systematic review by searching all eligible literature on the change in treatment strategies and obstructive sites found during DISE in pediatric conventional OSAHS. This research aims to explore the role of DISE in the management of pediatric conventional OSAHS.
Patients and methods
Search strategies
Two authors (YC. C. and X. W.) have conducted comprehensive search in PubMed, EMBASE, Cochrane Library, Web of Science, China National Knowledge Infrastructure (CNKI), WANFANG Data databases (WF), and VIP database (VIP) up to January 31, 2023, that investigate the role of DISE in the treatment of pediatric conventional OSAHS. We used the following search terms: “snoring,” “sleep apnea,” “obstructive sleep apnea hypopnea syndrome,” “OSAHS,” “obstructive sleep apnea,” “OSA,” “drug-induced sleep endoscopy,” “drug-induced sedation endoscopy,” “DISE,” “child,” “children,” “pediatric” separated by the Boolean operator AND or OR. Additionally, manual searching of selected literature was conducted.
Inclusion and exclusion criteria
The inclusion criteria were (1) original studies published in either Chinese or English; (2) studies involving pediatric participants diagnosed with OSAHS, with no restrictions on gender, ethnicity, geographic origin, disease severity, or duration; (3) children undergoing DISE prior to first-line treatment for OSAHS, with guidance on treatment; (4) the primary outcome is the frequency of treatment plan modifications after DISE, and secondary outcomes include identification of obstructed sites during DISE and treatment-related information.
The exclusion criteria were (1) publications in languages other than Chinese or English; (2) unpublished or incomplete original data, inability to obtain important data after contacting the authors; (3) general reviews or duplicate publications, or in the case of multiple publications targeting the same population, only the highest-quality or largest sample size publication was selected; (4) unclear diagnostic criteria, a history of upper airway surgeries such as adenotonsillectomy, or comorbidities such as Down syndrome, severe craniofacial anomalies, or neuromuscular diseases.
Study selection and data extraction
Two authors (YC. C. and X. W.) identified and screened potentially eligible studies by reviewing the title and abstract of each article. Relevant publications were then selected for full-text review. Studies not clear were discussed and decided whether excluding it. In the case of discrepancies in included study between investigators, a third investigator (YS. T.) made the definitive decision via discussion.
YC. C. and X. W., respectively, assessed the relevance of search results and extracted the basic information of included study in Excel format. Discrepancies were resolved by discussion that consisted until consensus was achieved. Data extraction included general information (first author, publication year, study design, sample size), demographic data (age, gender, BMI, etc.), intervention details (medications used in DISE), and outcome data (frequency of treatment plan modifications, obstructed sites identified during examination, treatment details). Data extraction followed the methods outlined in the Cochrane Handbook for Systematic Reviews [19].
Quality assessment of included studies
The Newcastle–Ottawa Scale (NOS) [20] was used to assess the quality of included studies. Criteria assessed included selection (0–4 points), comparability (0–2 points), and outcome/exposure (0–3 points).
Statistical analysis
Single-arm meta-analysis was conducted using Stata 17.0. Heterogeneity was assessed using the Q-test and I2-statistic. If P ≥ 0.10 and I2 ≤ 50.0%, indicating homogeneity among studies, a fixed-effects model was employed. Otherwise, a random-effects model was used, and subgroup and sensitivity analyses were performed to explore potential sources of heterogeneity (e.g., sample size). A 95% confidence interval (CI) was used for interval estimation, with a significance level of α = 0.05.
Results
Literature screening results
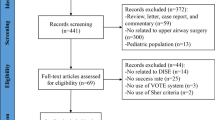
Following the retrieval strategy, a total of 425 articles were retrieved, distributed among various databases as follows: 146 from PubMed, 183 from Embase, 146 from Cochrane Library, 160 from Web of Science, 100 from CNKI, 131 from WF, and 110 from VIP. After deduplication and screening of titles, abstracts, and full texts, a strict adherence to the inclusion and exclusion criteria resulted in the final inclusion of 4 studies [10, 18, 21, 22], comprising a total of 761 pediatric cases. The literature screening process and outcomes are illustrated in Fig. 1. Notably, two publications by Chen et al. [21, 23] pertained to the same study population, and both were considered in the data analysis. Table 1 presents general information about the included studies and their quality assessment. The quality assessment revealed that the methodological quality of the included studies was generally low, as all were non-randomized case studies.
Meta-analysis results
Obstructed sites
All four studies reported on the obstructed sites identified during DISE in pediatric conventional OSAHS. However, the study by Gazzaz et al. [18] did not report on nasal and pharyngeal (adenoid) obstruction, while the study by Williamson et al. [10] did not report on soft palate obstruction. Based on the available data, a meta-analysis was conducted on the obstruction rates in the nasopharynx (adenoid), soft palate, oropharyngeal (tonsils), tongue base, and supraglottic levels. Due to heterogeneity among the studies, a random-effects model was used for all analyses. The forest plot (Fig. 2) illustrates the obstruction rates found during DISE: nasopharyngeal (adenoid) obstruction rate of 93% (95% CI: 85–100%), soft palate obstruction rate of 35% (95% CI: 14–55%), oropharyngeal (tonsil) obstruction rate of 76% (95% CI: 54–98%), tongue base obstruction rate of 32% (95% CI: 3–61%), and supraglottic obstruction rate of 31% (95% CI: 5–56%). Sensitivity analyses for each level showed that the exclusion of any single study did not lead to Rate values outside the 95% CI of the overall rate, indicating the robustness of the results.
Two studies [10, 11] provided information on multi-level obstruction (defined as obstruction in more than one level, including but not limited to adenoid and tonsil obstruction) in pediatric conventional OSAHS, involving a total of 77 cases. Since the heterogeneity test indicated clinical and statistical homogeneity among the studies (P = 0.61, I2 = 0.0%), a fixed-effects model was used. The forest plot (Fig. 3) shows a multi-level obstruction rate of 60% (95% CI: 49–71%).
Rate of treatment modification
A meta-analysis was conducted on the rate of treatment modification guided by drug-induced sleep endoscopy (DISE) in the 761 patients from the four studies. Due to significant heterogeneity among the studies (P = 0.000, I2 = 92.1%), a random-effects model was utilized. The forest plot (Fig. 4) shows that DISE resulted in treatment modification in 45% (95% CI: 29–60%) of pediatric conventional OSAHS. Subgroup analysis indicated that sample size and the use of medications during DISE had no impact on reducing heterogeneity. Sensitivity analysis (Fig. 5) demonstrated that excluding any single study did not result in rate values outside the 95% CI of the overall rate, reaffirming the reliability of the results.
Three studies provided specific descriptions of the treatment received by patients after DISE (Table 2). Adenoid and tonsillectomy were the primary treatment modalities for pediatric conventional OSAHS. However, some pediatric conventional OSAHS also had indications for tongue base surgery and supraglottic surgery after DISE.
Discussion
In recent years, DISE has garnered increasing attention and rapidly evolved as a novel technique for upper airway assessment. To date, there is a wide divergence of opinions regarding the indications for pediatric DISE. Nonetheless, the majority of medical professionals deem DISE suitable for persistent OSAHS after T&A and special populations, such as those with syndromes or neuromuscular abnormalities, and consensus guidelines have been developed for these scenarios [11, 13,14,15]. However, considerable controversy persists regarding the applicability of preoperative DISE for pediatric conventional OSAHS.
In this study, we employed a meta-analysis and systematic review approach to investigate the role of DISE in the treatment of pediatric conventional OSAHS. A total of four case studies were included, encompassing 761 study subjects who had no history of upper airway surgery, craniofacial anomalies, or syndromes. The quality assessment of the included studies indicated a relatively low methodological quality, as all were non-randomized case studies.
DISE serves as an effective diagnostic tool, capable of identifying obstructive sites that may be overlooked during routine ear, nose, and throat examinations of awake children. Furthermore, it aids in tailoring specific and effective treatment plans for children, thus minimizing the need for unnecessary surgeries [12]. Our findings within the cohort of routine OSAHS in children undergoing DISE revealed obstruction rates in various regions as follows: nasopharynx (adenoids) 93%, soft palate 35%, oropharynx (tonsils) 76%, base of tongue 32%, supraglottis 31%, and multi-level obstruction 60%. A systematic review conducted in 2016 demonstrated that at least one obstructive site was identified in 100% of post-tonsillectomy and adenoidectomy (T&A) persistent OSAHS children who underwent DISE evaluation. The base of the tongue was the most frequently obstructed site, with tongue base tonsillectomy and supraglottoplasty being the most commonly employed treatment methods [24]. These results emphasize the importance of assessing the impact of the soft palate, base of tongue, and supraglottis when evaluating obstruction levels in pediatric conventional OSAHS.
Our meta-analysis results indicate that DISE altered the conventional surgical approach in 45% (95% CI: 29–60%) of pediatric conventional OSAHS. Within the included studies, DISE provided personalized treatment plans for children, leading to significant improvements in postoperative symptoms and sleep-related parameters compared to preoperative conditions. This substantially increased the surgical success rate while avoiding unnecessary surgeries. Therefore, preoperative DISE in pediatric conventional OSAHS can effectively identify upper airway obstructions, reducing the likelihood of postoperative persistent OSAHS and the need for secondary surgeries. Boudewyns et al. [11] reported a surgical success rate of 91% in 22 patients with PSG data guided by DISE. Chen et al.‘s study demonstrated significant improvements in preoperative and postoperative RDI and lowest SaO2. Williamson et al.‘s research indicated that under DISE-guided treatment, symptoms significantly improved in 91.4% of children, with significant improvements in AHI and lowest SaO2 observed in 13 patients with PSG data [10].
The results of our meta-analysis were in favor of DISE-directed approach for pediatric OSAHS without previous upper airway surgery or comorbidity. In the context of expanding indications, DISE should be considered in these patient population. It is worth acknowledging that the good effect of T&A, and T&A attains a success rate within the range of 71 to 87% in healthy children with OSAHS [6, 25]. Nevertheless, it is crucial to recognize that factors such as obesity, small tonsil size, and severe AHI independently pose increased risks for postoperative persistence of OSAHS in children [10, 26]. In the presence of these independent risk factors for ongoing disease, DISE-directed approach should rather be recommended for them.
Currently, anesthesia medications that can be used for pediatric DISE, including propofol, dexmedetomidine, and midazolam, but each exhibiting distinct effects on respiratory physiology [14, 27,28,29]. Dexmedetomidine possesses sedative and analgesic properties while exerting minimal influence on respiratory depression [30, 31]. However, the use of propofol in DISE presents complexities due to its potential to induce muscle relaxation, decrease ventilatory drive, and lead to respiratory depression, particularly in patients with OSAHS [30, 31]. Among the included studies, pediatric patients undergoing DISE are typically sedated using either a continuous intravenous infusion of propofol or dexmedetomidine as the most common sedation options. The variability in obstruction patterns observed with different DISE medications is a consideration in the surgical decision-making process. In the context of adult DISE, certain comparative studies between dexmedetomidine and propofol have reported a higher incidence of respiratory depression and tongue base obstruction associated with propofol [32]. However, in accordance with the research findings presented by Kirkham et al. [33], there was no statistically significant difference in the likelihood of ≥ 50% obstruction during DISE when utilizing either dexmedetomidine or propofol at various anatomical levels within a cohort of 117 pediatric patients diagnosed with OSAHS. Similarly, we conducted subgroup analyses on the basis of medication status and that had no impact on reducing heterogeneity. Hence, we contend that minor variances in DISE medications would likely exert limited clinical repercussions in practice.
Several studies have also affirmed the role of DISE in the treatment of pediatric conventional OSAHS [13, 34,35,36,37,38]. However, some of these studies combined pediatric conventional OSAHS with postoperative persistent OSAHS and did not exclude patients with syndromes, resulting in significant heterogeneity among the study subjects. Therefore, they were not included in the meta-analysis. Galluzzi et al. [16] conducted a systematic review of the proportion of tonsillar and/or adenoid hypertrophy in children with OSAHS undergoing DISE, including five studies (n = 39). They estimated that tonsillar and/or adenoid hypertrophy accounted for OSAHS in 71% (95% CI: 64–77%) of cases and argued against the widespread use of DISE in children with OSAHS. However, their study suffered from substantial subject heterogeneity (including a significant number of syndrome patients) and did not compare the outcomes of surgeries guided by DISE with those guided by conventional surgical indications. Additionally, they did not provide a comparison of obstructive sites identified by DISE. Therefore, the conclusions of their study are questionable.
Limitations of this study should be acknowledged. First, the inclusion criteria were limited to studies published in Chinese and English, potentially resulting in the omission of relevant literature published in other languages, which could lead to incomplete coverage of the available literature. Second, the clinical evidence level within the included studies is relatively low, primarily due to the absence of high-quality, rigorously conducted randomized controlled trials (RCTs) incorporating blind methodologies. Due to the absence of randomized controls, our conclusions regarding DISE’s impact on outcomes and the avoidance of unnecessary procedures are speculative. Third, the scarcity of detailed information in the included studies hindered the ability to perform subgroup analyses based on factors such as BMI or tonsil size, potentially introducing significant clinical heterogeneity.
Conclusions
In some cases, DISE may potentially lead to alterations in conventional surgical approaches for children with OSAHS who had no history of upper airway surgery, craniofacial abnormalities, or other syndromes. The results of our meta-analysis were in favor of a DISE-directed approach for pediatric conventional OSAHS. However, further high-quality randomized controlled trials (RCTs) are warranted in future research to investigate the role of DISE in the management of pediatric OSAHS.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, Jones J, Schechter MS, Sheldon SH, Spruyt K, Ward SD et al (2012) Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 130(3):576–584
Li AM, So HK, Au CT, Ho C, Lau J, Ng SK, Abdullah VJ, Fok TF, Wing YK (2010) Epidemiology of obstructive sleep apnoea syndrome in Chinese children: a two-phase community study. Thorax 65(11):991–997
[Chinese guideline for the diagnosis and treatment of childhood obstructive sleep apnea (2020)] (2020) Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi = Chinese journal of otorhinolaryngology head and neck surgery 55(8):729–747. https://doi.org/10.3760/cma.j.cn115330-20200521-00431
Xiao L, Sunkonkit K, Shi J, Narang I (2023) Neurocognition, behavior, socioeconomic and health outcomes of pediatric obstructive sleep apnea. Am J Respir Crit Care Med 207(7):936–938. https://doi.org/10.1164/rccm.202201-0051RR
Bhattacharjee R, Kheirandish-Gozal L, Spruyt K, Mitchell RB, Promchiarak J, Simakajornboon N, Kaditis AG, Splaingard D, Splaingard M, Brooks LJ et al (2010) Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: a multicenter retrospective study. Am J Respir Crit Care Med 182(5):676–683
Mitchell RB (2007) Adenotonsillectomy for obstructive sleep apnea in children: outcome evaluated by pre- and postoperative polysomnography. Laryngoscope 117(10):1844–1854
Marcus CL, Moore RH, Rosen CL, Giordani B, Garetz SL, Taylor HG, Mitchell RB, Amin R, Katz ES, Arens R et al (2013) A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med 368(25):2366–2376
Tauman R, Gulliver TE, Krishna J, Montgomery-Downs HE, O’Brien LM, Ivanenko A, Gozal D (2006) Persistence of obstructive sleep apnea syndrome in children after adenotonsillectomy. J Pediatr 149(6):803–808
Friedman M, Wilson M, Lin HC, Chang HW (2009) Updated systematic review of tonsillectomy and adenoidectomy for treatment of pediatric obstructive sleep apnea/hypopnea syndrome. Otolaryngol--Head Neck Surg : official journal of American Academy of Otolaryngology-Head and Neck Surgery 140(6):800–808
Williamson A, Coutras SW, Carr MM (2022) Sleep endoscopy findings in children with obstructive sleep apnea and small tonsils. Ann Otol Rhinol Laryngol 131(8):851–858
Boudewyns A, Verhulst S, Maris M, Saldien V, Van de Heyning P (2014) Drug-induced sedation endoscopy in pediatric obstructive sleep apnea syndrome. Sleep Med 15(12):1526–1531
Wilcox LJ, Bergeron M, Reghunathan S, Ishman SL (2017) An updated review of pediatric drug-induced sleep endoscopy. Laryngoscope investigative otolaryngology 2(6):423–431
Raposo D, Menezes M, Rito J, Trindade-Soares M, Adónis C, Loureiro HC, Freire F (2021) Drug-induced sleep endoscopy in pediatric obstructive sleep apnea. Otolaryngol-Head and Neck Surg: official journal of American Academy of Otolaryngology-Head and Neck Surgery 164(2):414–421
Baldassari CM, Lam DJ, Ishman SL, Chernobilsky B, Friedman NR, Giordano T, Lawlor C, Mitchell RB, Nardone H, Ruda J et al (2021) Expert consensus statement: pediatric drug-induced sleep endoscopy. Otolaryngol—Head Neck Surg 194599820985000
Lan MC, Hsu YB, Lan MY, Chiu TJ, Huang TT, Wong SB, Chen YC, Tsai LP (2016) Drug-induced sleep endoscopy in children with Prader-Willi syndrome. Sleep Breathing = Schlaf Atmung 20(3):1029–1034
Galluzzi F, Pignataro L, Gaini RM, Garavello W (2015) Drug induced sleep endoscopy in the decision-making process of children with obstructive sleep apnea. Sleep Med 16(3):331–335
Boudewyns A, Verhulst S (2015) Potential role for drug-induced sleep endoscopy (DISE) in paediatric OSA. Sleep Med 16(9):1178
Gazzaz MJ, Isaac A, Anderson S, Alsufyani N, Alrajhi Y, El-Hakim H (2017) Does drug-induced sleep endoscopy change the surgical decision in surgically naïve non-syndromic children with snoring/sleep disordered breathing from the standard adenotonsillectomy? A retrospective cohort study. J Otolaryngol - Head Neck Surg = Le Journal d’oto-rhino-laryngologie et de chirurgie cervico-faciale 46(1):12
Higgins JPT, Green S (2008) Cochrane handbook for systematic reviews of interventions version 5.0.1. The Cochrane Collaboration. https://www.cochrane-handbook.org
GA Wells BS, D O'Connell, J Peterson, V Welch, M Losos, P Tugwell (2019) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. In: http://www.ohrica/programs/clinical_epidemiology/oxfordasp. edn. 2019–03–17
Chen J, He S (2019) Drug-induced sleep endoscopy-directed adenotonsillectomy in pediatric obstructive sleep apnea with small tonsils. PLoS ONE 14(2):e0212317
Boudewyns A, Saldien V, Van de Heyning P, Verhulst S (2018) Drug-induced sedation endoscopy in surgically naïve infants and children with obstructive sleep apnea: impact on treatment decision and outcome. Sleep Breathing = Schlaf Atmung 22(2):503–510
He S, Zhang R, Ma S, Chen J (2020) Comparison and intervention of differences in upper airway obstruction in children with OSAS between awake and asleep. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 34(8):713–718
Manickam PV, Shott SR, Boss EF, Cohen AP, Meinzen-Derr JK, Amin RS, Ishman SL (2016) Systematic review of site of obstruction identification and non-CPAP treatment options for children with persistent pediatric obstructive sleep apnea. Laryngoscope 126(2):491–500
Ye J, Liu H, Zhang GH, Li P, Yang QT, Liu X, Li Y (2010) Outcome of adenotonsillectomy for obstructive sleep apnea syndrome in children. Ann Otol Rhinol Laryngol 119(8):506–513
Lee CH, Hsu WC, Chang WH, Lin MT, Kang KT (2016) Polysomnographic findings after adenotonsillectomy for obstructive sleep apnoea in obese and non-obese children: a systematic review and meta-analysis. Clin Otolaryngol : official journal of ENT-UK; official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery 41(5):498–510
Liu KA, Liu CC, Alex G, Szmuk P, Mitchell RB (2020) Anesthetic management of children undergoing drug-induced sleep endoscopy: a retrospective review. Int J Pediatr Otorhinolaryngol 139:110440
Ehsan ZMM, Shott SR et al (2016) The effects of anesthesia and opioids on the upper airway: a systematic review. Laryngoscope 126(1):270–284
Friedman NR, Parikh SR, Ishman SL, Ruiz AG, El-Hakim H, Ulualp SO, Wootten CT, Koltai PJ, Chan DK (2017) The current state of pediatric drug-induced sleep endoscopy. Laryngoscope 127(1):266–272
Padiyara TV, Bansal S, Jain D, Arora S, Gandhi K (2020) Dexmedetomidine versus propofol at different sedation depths during drug-induced sleep endoscopy: a randomized trial. Laryngoscope 130(1):257–262
Ordones AB, Grad GF, Cahali MB, Lorenzi-Filho G, Sennes LU, Genta PR (2020) Comparison of upper airway obstruction during zolpidem-induced sleep and propofol-induced sleep in patients with obstructive sleep apnea: a pilot study. J Clin Sleep Med: JCSM : official publication of the American Academy of Sleep Medicine 16(5):725–732
Capasso R, Rosa T, Tsou DY, Nekhendzy V, Drover D, Collins J, Zaghi S, Camacho M (2016) Variable findings for drug-induced sleep endoscopy in obstructive sleep apnea with propofol versus dexmedetomidine. Otolaryngol--Head Neck Surg official journal of American Academy of Otolaryngology-Head and Neck Surgery 154(4):765–770
Kirkham EM, Hoi K, Melendez JB, Henderson LM, Leis AM, Puglia MP 2nd, Chervin RD (2021) Propofol versus dexmedetomidine during drug-induced sleep endoscopy (DISE) for pediatric obstructive sleep apnea. Sleep breathing = Schlaf Atmung 25(2):757–765
Frederick RM 2nd, Brandt J, Sheyn A (2022) Drug-induced sleep endoscopy effect on intraoperative decision making in pediatric sleep surgery: a 2-year follow up. Laryngoscope Investig Otolaryngol 7(6):2112–2118
Filipek N, Kirkham E, Chen M, Ma CC, Horn DL, Johnson KE, Parikh SR (2021) Drug-induced sleep endoscopy directed surgery improves polysomnography measures in overweight and obese children with obstructive sleep apnea. Acta Otolaryngol 141(4):397–402
Collu MA, Esteller E, Lipari F, Haspert R, Mulas D, Diaz MA, Dwivedi RC (2018) A case-control study of drug-induced sleep endoscopy (DISE) in pediatric population: a proposal for indications. Int J Pediatr Otorhinolaryngol 108:113–119
Blanc F, Kennel T, Merklen F, Blanchet C, Mondain M, Akkari M (2019) Contribution of drug-induced sleep endoscopy to the management of pediatric obstructive sleep apnea/hypopnea syndrome. Eur Ann Otorhinolaryngol Head Neck Dis 136(6):447–454
Miller C, Kirkham E, Ma CC, Filipek N, Horn DL, Johnson K, Chen ML, Parikh SR (2019) Polysomnography outcomes in children with small tonsils undergoing drug-induced sleep endoscopy-directed surgery. Laryngoscope 129(12):2771–2774
Funding
This work was supported by the Shenzhen Municipal Science and Technology Innovation Committee (no. JCYJ20210324143008022) and Guangdong High-level Hospital Construction Fund Clinical Research Project of Shenzhen Children’s Hospital (no. LCYJ2022063).
Author information
Authors and Affiliations
Contributions
All the authors contributed significantly to this work. YC. C. and X. W. designed the research study; YC. C., X. W., and YS. T. performed the research study and extracted and analyzed the data;, YC. C., X. W., L. L., HG. P., and YS. T. wrote and revised the manuscript. All authors provided final approval and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Research involving human participants and/or animals
For this type of study (systematic review), formal consent is not applicable.
Informed consent
For this type of study (systematic review), formal consent is not applicable.
Additional information
This article does not contain any studies with human participants performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xin Wang and Yong-chao Chen have contributed equally to this work and share first authorship.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, X., Chen, Yc., Li, L. et al. Effects of drug-induced sleep endoscopy in children with conventional obstructive sleep apnea–hypopnea syndrome: a systematic review and meta-analysis. Sleep Breath 28, 935–944 (2024). https://doi.org/10.1007/s11325-023-02945-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-023-02945-7