Abstract
Purpose
Central sleep apnea (CSA) is usually distinguished from obstructive sleep apnea (OSA). In fact, CSA is often a component of severe sleep apnea hypopnea syndrome (SAHS), rather than occurring alone. We investigated the clinical characteristics and polysomnography (PSG) parameters of CSA components in patients with severe SAHS.
Methods
The clinical characteristics and PSG parameters were retrospectively analyzed.
Results
Pure or dominant CSA was rare (5% of all patients). Of all patients with CSA, 72% also exhibited other apnea subtypes that contributed to severe SAHS. Among patients with severe SAHS, those with CSA were more likely than others to be older; thinner; exhibit higher prevalences of comorbid coronary heart disease, arrhythmia, and heart failure; a higher apnea/hypopnea index (AHI); mixed apnea index (MAI); an elevated oxygen desaturation index (ODI); and more nighttime oxygen saturation levels < 90%. Multivariate logistic regression analysis revealed that older age, comorbid arrhythmia or heart failure, and an elevated ODI were independently associated with CSA.
Conclusion
Patients who complain of snoring or apnea may be better evaluated by comprehensive PSG prior to treatment if they are old, show greater hypoxia, or suffer from arrhythmia and/or heart failure, because such patients are more likely than others to exhibit CSA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is very common and is characterized by recurrent episodes of upper airway collapse leading to complete or partial cessation of airflow even with respiratory effort [1]. By contrast, central sleep apnea (CSA), characterized by respiratory apnea accompanied by a lack of respiratory effort during sleep, is much less common, except in patients with cardiac diseases or those who take opiates [2,3,4]. Previous studies have focused on patients with dominant CSA, regardless of the severity of sleep apnea hypopnea syndrome (SAHS) [5,6,7]. However, it is noteworthy that CSA is typically comorbid with OSA, mixed sleep apnea (MSA), and hypopnea. These types of SAHS may constitute a severe phenotype (the apnea/hypopnea index [AHI] is ≥ 30) together, which may be more harmful and deserving treatment. Continuous positive airway pressure ventilation (CPAP) may be used to treat suspected OSA directly. But for those patients with a component of CSA, there may be a greater possibility of complex sleep apnea syndrome or lower adherence to CPAP therapy [8,9,10]. The home sleep apnea test (HSAT) detects OSA in a cost-effective manner but does not distinguish CSA [8, 11]. To allocate medical resources optimally and to ensure appropriate treatment, it is important to be alert for CSA in patients with SAHS irrespective of which apnea subtype predominates. We therefore explored the clinical characteristics and PSG parameters of patients with severe SAHS with and without CSA regardless of whether or not CSA predominated. We also sought indicators of CSA events in an effort to facilitate appropriate diagnosis and treatment.
Methods
Subjects
Patients who complained of snoring or apnea and who underwent overnight PSG in the Sleep Center of Tianjin Chest Hospital were retrospectively enrolled from January 2015 to March 2022. The exclusion criteria were total sleep time < 120 min, long-term sedative or sleeping tablet usage, any prior SAHS diagnosis, and noninvasive positive pressure ventilation treatment. We defined pure CSA as a central apnea index (CAI) ≥ 5 with a respiratory event index of any/all other subtype(s) < 5; dominant CSA as an AHI ≥ 5 and a CAI-to-AHI ratio > 50%; and secondary CSA as an AHI ≥ 5 and a CAI ≥ 5 and a CAI-to-AHI ratio ≤ 50%. When exploring the contributions of CAI to severe SAHS, we divided patients into a wCSA group (AHI ≥ 30 and CAI ≥ 5) and an nCSA group (AHI ≥ 30 and CAI < 5). We recorded sex, age, height, weight, neck and waist circumference, and comorbidities. The body mass index (BMI) was the weight in kilograms divided by the square of the height in meters. Atrial arrhythmia was defined as persistent atrial fibrillation (AF) or atrial flutter. Heart failure was defined by a previous diagnosis via echocardiography, assay of the brain natriuretic peptide (BNP) level, or obvious symptoms.
PSG
Overnight PSG yielded electroencephalography data (F4/M1, C4/M1, O2/M1, F3/M2, C3/M2, O1/M2) and also included electrooculography, submental electromyography, bilateral anterior tibialis electromyography, and electrocardiography. Respiratory inductance plethysmography was used to monitor the respiratory effort of thoracoabdominal movement. Airflow was assessed using oronasal thermal and pressure sensors. A finger pulse oximeter was employed to record oxygen saturation. Sleep stages were manually assessed using the 2012 American Academy Sleep Medicine criteria [4]. Apnea was defined as an airflow reduction > 90% of the pre-event baseline for ≥ 10 s; hypopnea was defined as a reduction in airflow ≥ 30% of the pre-event baseline for ≥ 10 s with ≥ 3% oxygen desaturation. OSA and CSA events were defined as apneas in the presence or absence of respiratory effort, respectively. Mild, moderate, and severe SAHS were defined as 5 ≤ AHI < 15 events/h, 15 ≤ AHI < 30 events/h, and AHI ≥ 30 events/h, respectively. The oxygen desaturation index (ODI) was the number of times in which oxygen saturation decreased by 3%/h.
Statistical analysis
Normally distributed quantitative data are presented as means ± standard deviations and non-normally distributed data are given as medians with interquartile ranges. Qualitative data are expressed as frequencies with percentages. Numerical variables were compared using the independent paired t-test and the Mann-Whitney U-test for normally and non-normally distributed data, respectively. Categorical variables were compared employing the chi-square or Fisher exact test (as appropriate). Only variables with P-values < 0.1 were included in multivariate logistic regression, except cerebrovascular disease. In view of the difference in the prevalence of cerebrovascular disease between the two groups and the important influence of cerebrovascular disease on CSA, cerebrovascular disease (P = 0.324) was also included in the multivariate regression analysis. For all analyses, a two-tailed P < 0.05 was considered significant. All analyses were performed using SPSS ver. 25 software.
Results
Subtypes of patients who underwent PSG
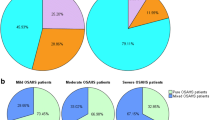
Of 714 patients who met the inclusion criteria, 123 (17%) had CSA and 3 (2%), 13 (11%), and 107 (87%) for mild, moderate, and severe SAHS, respectively. In all, 10 patients (8%) had pure CSA, 24 (20%) had dominant CSA, and 89 (72%) had secondary CSA (Figure 1A). Thus, most CSA events were comorbid with other apnea subtypes; such combinations constituted severe SAHS. In 553 patients with severe SAHS, 107 (19%) exhibited CSA (CAI ≥ 5, defined as wCSA group) but 446 (80%) did not (CAI < 5, defined as nCSA group). In the wCSA group with severe SAHS, dominant CSA accounted for only 20% of patients (21/107). Among patients with severe SAHS, both with and without CSA, obstructive apneas were the most common type of events (Figure 1B).
Apnea subtypes of SAHS patients, and SAHS severities. A Secondary CSA patients were much more common than pure and dominant CSA patients. The severe SAHS phenotype predominated in both the dominant and secondary CSA groups. B 553 patients with severe SAHS (AHI≥30, dominant subtype unspecified) were divided into wCSA group and nCSA group according to CSA existed (CAI≥5) or not. Patients lacking CSA (nCSA) were much more common than those with CSA (wCSA). The OSA subtype predominated in both groups, followed by hypopnea (HYP), and then MSA, grouping variable CSA not included
Characteristics, comorbidities, and PSG parameters
We included 553 patients with severe SAHS. Most were males (466, 84%) of mean age 52.7 ± 12.1 years with a median AHI of 51.6 (39.2, 64.4)/h. Patients with (compared to without) CSA were older, thinner, and more likely to exhibit comorbid coronary heart disease, arrhythmia, and heart failure (all P < 0.05). Sex and neck and waist circumference did not differ between the two groups; neither did the prevalence of diabetes, cerebrovascular or chronic airway inflammatory disease, or pulmonary embolism (Table 1). Compared to the nCSA group, wCSA patients had a higher AHI and MAI but a lower OAI and hypopnea index (HI). The REM and NREM percentages of total sleep time (TST) did not significantly differ. The percent of night oxygen saturation < 90% (Tsat90) was somewhat greater in the wCSA group, but the ODI was significantly higher (Table 2).
Correlations between the CSA and other parameters
Only variables with P-values < 0.1 in univariate analysis were included in multivariate logistic regression analysis, with the exceptions of the MAI and HI (only the AHI was included because the AHI is strongly associated with the MAI, CAI, and HI), and exceptions of cerebrovascular disease due to the reasons mentioned above. Older age, a greater ODI, arrhythmia, and heart failure independently predicted comorbid CSA in patients with severe SAHS (Table 3). The area under the receiver operator curves for these four variables (combined) was 0.690 (95% confidence interval 0.634–0.747, P < 0.001). The predictive sensitivity was 62.3% and the specificity was 68.3% (Figure 2).
Discussion
We found that true CSA (defined as pure and dominant CSA in this article) was rare; the prevalence was 5% (34/714) in our general cohort. The majority of patients with CAIs ≥ 5 exhibited complex phenotypes with CSA components; they had severe SAHS. Patients with CSA differed from others in terms of age, BMI, comorbidities (coronary heart disease, arrhythmia, heart failure), the respiratory event indices, and the extent of oxygen desaturation.
A retrospective study in military veterans indicated that the prevalence of CSA was 0.6%, of which 64.1% was comorbid with OSA [5]. Lucas et al. reported a CSA prevalence of 0.9% in a community population [7], but 1.8% for males and 2.7% for males aged ≥ 65 years. Bixler et al. studied community males; the CSA prevalence was low (0.4%) but increased monotonically with age [12]. A clinical study reported a primary CSA prevalence of 3.8%, 64% of which was severe [6]. The prevalence has varied across studies. Clinical studies have reported higher prevalences than community-based works. Several studies have found that CSA patients are more likely to be male than those with OSA [5, 12, 13]. In our study, males were much more common than females in both groups, but the proportions were similar in the two groups (85.0 vs. 84.1%, P = 0.805), perhaps because wCSA features many OSA components. Several studies have found that CSA patients are thinner than controls [5, 7]. We similarly found that the BMI of the wCSA group was lower than that of the nCSA group (28.76 vs. 29.70 kg/m2, P = 0.026).
OSA increases the risk for and progression of hypertension, type 2 diabetes, cardiovascular stroke, and atrial fibrillation [1, 14,15,16,17]. Strong associations between CSA and arrhythmias and heart failure have recently been reported. Harmon et al. found that the prevalence of OSA in AF and non-AF groups was similar (54.7 vs. 52.0%, P = 0.56) [18]. CSA was more common in their AF group (12.3 vs. 4.4%, P = 0.002). A prospective study showed that CSA (odds ratio [OR] 2.58, 95% CI 1.18–5.66) and CSA with Cheyne-Stokes breath (CSB) (OR 2.27, 95% CI 1.13–4.56), but not OSA, predicted incident atrial fibrillation [19]. The association was much stronger in patients aged ≥ 76 years. Another study drew similar conclusions: CSA and CSB were significantly associated with AF (OR 5.15, 95% CI 2.21–12.52 and OR 6.26, 95% CI 2.05–19.14, respectively) [20]. However, OSA was not significantly associated with AF. Grimm et al. found that the prevalence of CSA (AHI ≥ 15) was 43% in a cohort with left ventricular ejection fraction (LVEF) ≤ 50%. AF was strongly associated with severe CSA [21]. In a cohort of heart-failure patients with reduced or preserved LVEF, the LVEF correlated negatively with the CAI (r = −0.558, P < 0.001) but not with the OAI [22]. Thus, CSA is associated more strongly with AF and HF than OSA, despite the high prevalence of OSA in AF and HF patients, perhaps because OSA per se is common in general populations. Our data are consistent with prior data. Compared to patients lacking CSA, the prevalences of arrhythmia (21 vs. 7.8%, P < 0.001) and heart failure (5 vs. 0.4%, P < 0.001) were higher in those with CSA.
In groups with and without CSA, OSA was the major component, and (unsurprisingly) the OAI was higher in the nCSA group. Notably, the MAI was significantly higher in the wCSA group, indicating a strong association with CSA. Mixed apnea features both central and obstructive components, and is usually considered an obstructive event [23]. Yamauchi et al. concluded that MSA is more closely associated with CSA than OSA, as revealed by instability of respiratory control; the cited authors studied respiratory signals and CPAP acceptance and compliance [24]. Yang et al. [25] found that MSA is linked to both a reduced mean oxygen saturation and the lowest oxygen saturation recorded, similar to what we found. Therefore, we hypothesize that both CSA and MSA are related to respiratory control unstable. We plan to investigate the potential for correlations between CSA and MSA events, and the loop gain and awakening threshold.
Regrettably, we did not categorize hypopnea into central or obstructive hypopnea using the 2012 Criteria of the American Academy of Sleep Medicine. In the clinic, we find it difficult to handle the inspiratory flattening/thoracoabdominal paradox. We may thus have underestimated central events. Central or obstructive events are optimally and precisely detected via esophageal, electrode catheter manometry [26], but patients dislike this.
As is true of all retrospective investigations, some patient data were lacking. In addition, selection bias may be in play; this would overestimate CSA events. However, this is the first work to view CSA events as components of severe SAHS; we did not focus on pure CSA. Patients with and without CA differed in terms of demography, complications, and PSG parameters. Physicians should have raised suspicion when patients are old, exhibit low oxygen saturation during sleep, and are comorbid with arrhythmia or heart failure.
In conclusion, CSA patients (compared to others) were older, more hypoxic, and more likely to suffer from arrhythmia and heart failure. Both MSA and CSA may be related to respiratory control instability. We recommend that patients with the above-mentioned risk factors undergo PSG rather than HSAT for the accurate diagnosis and appropriate therapy.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Franklin KA, Lindberg E (2015) Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis 7(8):1311–22. https://doi.org/10.3978/j.issn.2072-1439.2015.06.11
Rana AM, Sankari A. (2022) Central sleep apnea. StatPearls, Treasure Island
Eckert DJ, Jordan AS, Merchia P et al (2007) Central sleep apnea: pathophysiology and treatment. Chest 131(2):595–607. https://doi.org/10.1378/chest.06.2287
Berry RB, Budhiraja R, Gottlieb DJ et al (2012) Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events Deliberations of the sleep apnea definitions task force of the American Academy of Sleep Medicine. J Clin Sleep Med 8(5):597–619. https://doi.org/10.5664/jcsm.2172
Ratz D, Wiitala W, Badr MS et al (2018) Correlates and consequences of central sleep apnea in a national sample of US veterans. Sleep 08(41):9. https://doi.org/10.1093/sleep/zsy058
Kouri I, Kolla BP, Morgenthaler TI et al (2020) Frequency and outcomes of primary central sleep apnea in a population-based study. Sleep Med 68177-83. https://doi.org/10.1016/j.sleep.2019.12.008
Donovan LM, Kapur VK (2016) Prevalence and characteristics of central compared to obstructive sleep apnea: analyses from the sleep heart health study cohort. Sleep 39(7):1353–9. https://doi.org/10.5665/sleep.5962
Morgenthaler TI, Malhotra A, Berry RB et al (2021) Optimal NIV medicare access promotion: patients with central sleep apnea: a technical expert panel report from the American College of Chest Physicians, the American Association for Respiratory Care, the American Academy of Sleep Medicine, and the American Thoracic Society. Chest 160(5):e419–e25. https://doi.org/10.1016/j.chest.2021.07.039
Morgenthaler TI, Kagramanov V, Hanak V et al (2006) Complex sleep apnea syndrome: is it a unique clinical syndrome? Sleep 29(9):1203–9. https://doi.org/10.1093/sleep/29.9.1203
Fashanu OS, Quan SF (2022) Factors associated with treatment outcomes after use of auto-titrating CPAP therapy in adults with obstructive sleep apnea. Sleep Breath. https://doi.org/10.1007/s11325-022-02590-6
Kapur VK, Auckley DH, Chowdhuri S et al (2017) Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med 13(3):479–504. https://doi.org/10.5664/jcsm.6506
Bixler EO, Vgontzas AN, Ten Have T et al (1998) Effects of age on sleep apnea in men: I Prevalence and severity. Am J Respir Crit Care Med 157(1):144–8. https://doi.org/10.1164/ajrccm.157.1.9706079
Bixler EO, Vgontzas AN, Lin HM et al (2001) Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med 163(3 Pt 1):608–13. https://doi.org/10.1164/ajrccm.163.3.9911064
Mostafa SA, Mena SC, Antza C et al (2022) Sleep behaviours and associated habits and the progression of pre-diabetes to type 2 diabetes mellitus in adults: a systematic review and meta-analysis. Diab Vasc Dis Res 19(3):14791641221088824. https://doi.org/10.1177/14791641221088824
May AM, Van Wagoner DR, Mehra R (2017) OSA and cardiac arrhythmogenesis: mechanistic insights. Chest 151(1):225–41. https://doi.org/10.1016/j.chest.2016.09.014
Huang B, Liu H, Scherlag BJ et al (2021) Atrial fibrillation in obstructive sleep apnea: neural mechanisms and emerging therapies. Trends Cardiovasc Med 31(2):127–32. https://doi.org/10.1016/j.tcm.2020.01.006
Linz B, Hohl M, Lang L et al (2021) Repeated exposure to transient obstructive sleep apnea-related conditions causes an atrial fibrillation substrate in a chronic rat model. Heart Rhythm 18(3):455–64. https://doi.org/10.1016/j.hrthm.2020.10.011
Harmon EK, Stafford P, Ibrahim S et al (2020) Atrial fibrillation is associated with central sleep apnea in clinic patients undergoing diagnostic polysomnography. J Arrhythm 36(6):991–6. https://doi.org/10.1002/joa3.12427
May AM, Blackwell T, Stone PH et al (2016) Central sleep-disordered breathing predicts incident atrial fibrillation in older men. Am J Respir Crit Care Med 193(7):783–91. https://doi.org/10.1164/rccm.201508-1523OC
Anzai T, Grandinetti A, Katz AR et al (2020) Association between central sleep apnea and atrial fibrillation/flutter in Japanese-American men: the Kuakini Honolulu Heart Program (HHP) and Honolulu-Asia Aging Study (HAAS). J Electrocardiol 6110-7. https://doi.org/10.1016/j.jelectrocard.2020.05.005
Grimm W, Sass J, Sibai E et al (2015) Severe central sleep apnea is associated with atrial fibrillation in patients with left ventricular systolic dysfunction. Pacing Clin Electrophysiol 38(6):706–12. https://doi.org/10.1111/pace.12495
Wang T, Yu FC, Wei Q et al (2022) Prevalence and clinical characteristics of sleep-disordered breathing in patients with heart failure of different left ventricular ejection fractions. Sleep Breath. https://doi.org/10.1007/s11325-022-02611-4
Sleep-related breathing disorders in adults: (1999) recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 22(5):667-89
Yamauchi M, Tamaki S, Yoshikawa M et al (2011) Differences in breathing patterning during wakefulness in patients with mixed apnea-dominant vs obstructive-dominant sleep apnea. Chest 140(1):54–61. https://doi.org/10.1378/chest.10-1082
Yang X, Xiao Y, Han B et al (2019) Implication of mixed sleep apnea events in adult patients with obstructive sleep apnea-hypopnea syndrome. Sleep Breath 23(2):559–65. https://doi.org/10.1007/s11325-018-1745-0
Luo YM, Tang J, Jolley C et al (2009) Distinguishing obstructive from central sleep apnea events: diaphragm electromyogram and esophageal pressure compared. Chest 135(5):1133–41. https://doi.org/10.1378/chest.08-1695
Acknowledgements
We are grateful for all the subjects who were included in the study and all the staff in the Sleep Center of Tianjin Chest Hospital.
Funding
This project was funded by Major Research Plan of National Natural Science Foundation of China (Grant No. 92163213), General Program of National Natural Science Foundation of China (Grant No. 81970085), Tianjin science and technology plan project (Grant No. 21JCZDJC00940), Tianjin health science and technology projects (Grant No. TJWJ2022XK001), Tianjin Key Medical Discipline (Specialty) Construction Project (Grant No.TJYXZDXK-006A) and Tianjin Key Medical Discipline (specialty) Construction Project (Grant No. TJYXZDXK-049A).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval and consent to participate
All procedures performed in this study involving human subjects were in accordance with the ethical standards of the Ethics Committee of the Tianjin Chest Hospital and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, G., Zhao, X., Zhao, F. et al. Contribution of central sleep apnea to severe sleep apnea hypopnea syndrome. Sleep Breath 27, 1839–1845 (2023). https://doi.org/10.1007/s11325-023-02776-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-023-02776-6