Abstract
Purpose
The objective was to analyze the clinical implications of manual scoring of sleep studies using peripheral arterial tonometry (PAT) and to compare the manual and automated scoring algorithms.
Methods
Patients with suspected sleep-disordered breathing underwent sleep studies using PAT. The recordings were analyzed using a validated automated computer-based scoring and a novel manual scoring algorithm. The two methods were compared regarding sleep stages and respiratory events.
Results
Recordings of 130 patients were compared. The sleep stages and time were not significantly different between the scoring methods. PAT-derived apnea-hypopnea index (pAHI) was on average 8.4 events/h lower in the manually scored data (27.5±17.4/h vs.19.1±15.2/h, p<0.001). The OSA severity classification decreased in 66 (51%) of 130 recordings. A similar effect was found for the PAT-derived respiratory disturbance index with a reduction from 31.2±16.5/h to 21.7±14.4/h (p<0.001), for automated and manual scoring, respectively. A lower pAHI for manual scoring was found in all body positions and sleep stages and was independent of gender and body mass index. The absolute difference of pAHI increased with sleep apnea severity, while the relative difference decreased. Pearson’s correlation coefficient between pAHI and oxygen desaturation index (ODI) significantly improved from 0.89 to 0.94 with manual scoring (p<0.001).
Conclusions
Manual scoring results in a lower pAHI while improving the correlation to ODI. With manual scoring, the OSA category decreases in a clinically relevant proportion of patients. Sleep stages and time do not change significantly with manual scoring. In the authors’ opinion, manual oversight is recommended if clinical decisions are likely to change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is characterized by repetitive collapse of the upper airway resulting in arousals and sleep fragmentation [1]. This leads to disturbances in many biological processes and is associated with a higher risk for hypertension, heart failure, stroke, diabetes, and other diseases [2,3,4]. The prevalence of OSA, defined by an apnea-hypopnea index (AHI) greater than 15/h, is estimated to be as high as 23.4% in women and 49% in men [5]. Due to its high prevalence and associated complications, OSA has major socioeconomic relevance. However, it is believed that 93% of women and 83% of men with OSA remain undiagnosed and thus untreated [6]. Therefore, it is important to offer a cost-effective and reliable diagnosis of OSA.
Currently, sleep laboratory testing using polysomnography is the gold standard for diagnosing OSA. However, home sleep apnea testing (HSAT) offers the possibility for cost-effective and accurate assessment of OSA in selected patients [7]. It is less resource intensive and allows assessment of a patient’s sleep in his habitual sleeping environment. Furthermore, multiple-night testing can be performed to reduce the night-to-night variability and the first night effect [8].
Peripheral arterial tonometry (PAT) is a novel technique for HSAT and categorized as a Type 3 device together with respiratory polygraphy according to the American Academy of Sleep Medicine [7]. The PAT device is wrist worn and includes an accelerometer to detect movement. A finger probe measures the PAT signal and oxygen saturation. A chest sensor detects body position and includes a microphone to record an audio signal. This device setup has a low technical failure rate of 5.3% for at-home measurements [9].
In polysomnography, the cortical arousals from respiratory events are directly measured using electroencephalography. These cortical arousals also result in sympathetic activation and cause vasoconstriction mediated by alpha receptors. This vasoconstriction is measured in the finger to detect arousals of the autonomic nervous system. During these autonomic arousals, the PAT signal is attenuated, the heart rate increases, and the oxygen saturation decreases. Autonomic arousals are therefore a surrogate marker for cortical arousals when using electroencephalography [10].
PAT devices combine this information on arterial pulsatile arterial volume changes with heart rate variability, oxygen saturation, body position, and actigraphy to infer sleep-related breathing disturbances and sleep stages with their characteristic patterns. Since the PAT device does not measure airflow, all respiratory events are indirectly detected. To distinguish between indirectly and directly observed events, PAT events are referred to as peripheral arterial tonometry–derived apnea-hypopnea index (pAHI) and peripheral arterial tonometry–derived respiratory disturbance index (pRDI).
Previously, PAT recordings could only be analyzed using a proprietary, computer-based algorithm. This algorithm has been well validated for sleep-related breathing events and sleep stages [11, 12]. However, previously, no insight into the raw data has been possible. A novel software (zzzPAT® Itamar Medical, Caesarea, Israel) allows for manual scoring with visual oversight over the raw data of WatchPAT® recordings analogously to scoring for respiratory polygraphy or polysomnography. Zhang et al. developed an algorithm for manual oversight in an unselected patient cohort and could demonstrate that manual scoring improves the accuracy of sleep stages and respiratory events indices against polysomnography [10]. After the automated analysis is generated by the computer, the recordings are manually reviewed using visual oversight of the raw signals. First sleep stages and second respiratory events are classified by following an algorithm developed by Zhang et al. (further described in the “Methods”) [10].
To the knowledge of the authors, the manual algorithm has never been independently analyzed. It has been developed in an unselected patient collective and its effect on recordings performed at home and of patients with OSA is unclear. This article aims to analyze the clinical impact of manual scoring on sleep study results, such as pAHI or OSA severity classification. To answer this, we compared the results of automated and manual scoring and evaluated the clinical use of manual oversight of PAT recordings.
Methods
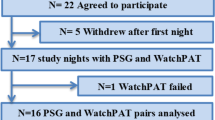
For this study, we retrospectively reviewed data of patients who were referred for suspected obstructive sleep apnea to our ear, nose, and throat (ENT) clinic between 2017 and 2020 for further evaluation. The cohort was an unselected patient collective, which was referred to our ENT clinic specializing in sleep medicine because of suspected OSA. OSA was suspected by the referring colleagues either based on history with typical OSA symptoms, screening questionnaires, or pathological pulse oximetry. All recordings were performed as screening and diagnostic workup before treatment. Only recordings of patients who consented to the use of their data were included and the study has been approved by the local ethics committee. Basic anthropomorphic data, such as height, weight, age, and gender, were collected. All recordings were performed at home using the WatchPAT® 200 device (Itamar Medical, Caesarea, Israel). Recordings with less than 4 h of sleep time in the automated analysis were excluded from the analysis. The PAT recordings were automatically scored using the proprietary validated computer-based scoring algorithm for WatchPAT® scoring in the zzzPAT® software (Itamar Medical, Caesarea, Israel). The automated algorithm was configured with a cut-off value of 3% oxygen desaturation for respiratory events as recommended by the American Academy of Sleep Medicine [7]. The oxygen desaturation index (ODI) was calculated using the default setting of 4% desaturation, which cannot be changed by the user. Manual scoring was performed according to the novel guidelines for manual scoring of PAT with the zzzPAT® software (Itamar Medical, Caesarea, Israel) by an experienced sleep technician or the authors [10]. After the automated analysis was generated by the computer, sleep stages and respiratory events were reviewed by visual overview of the raw signals. Respiratory events were deleted if no reduction in the PAT signal with a corresponding increase in heart rate was observed or if they were associated with positional change. Events were also deleted if there was a desaturation of less than 3% and no snoring pattern changes were observed. Events were added if a reciprocal pattern of PAT signal reduction and heart rate increase was observed with a greater than 3% desaturation. In REM sleep, desaturations of greater than 4% were marked as events.
The data was analyzed for systematic differences between automated and manual scoring regarding pAHI, pRDI, and ODI over the whole night and in different sleep stages and body positions. Furthermore, the classification of OSA severity was compared between automated and manual scoring. OSA severity was classified with pAHI as no OSA <5/h, mild 5 ≤ 15/h, moderate 15 ≤ 30/h, and severe >30/h. Lastly, time and proportion of sleep in different sleep stages were compared. For positional OSA, the ratio between pAHI in supine and non-supine position was calculated (Cartwright index) [13]. The REM association, analogously, is the ratio between pAHI in REM and NREM sleep [14]. Oxygen saturation and time in different body positions were calculated, but no comparison of automated and manual scoring was performed since these parameters do not require manual editing. However, differences between automated and manual scoring may arise due to manual adjustment of sleep and wake times as well as individual sleep stages.
Since no simultaneous other recording methods, such as polysomnography, were performed, we compared the correlation of pAHI and ODI with both scoring methods. We used this correlation as a surrogate marker for improved accuracy because many publications find a linear relationship with ODI and AHI as well as ODI and RDI [15,16,17]. Since no simultaneous measurements were performed, this is a surrogate marker, and the focus of this article is the effect of manual scoring and not its accuracy.
Normal distributed data were analyzed using Student’s t-tests and ANOVA for multiple groups and for nonnormal distributed data Wilcoxon’s rank-sum test or Kruskal-Wallis’ test was used. Correlations were calculated using the Pearson correlation. RStudio (Boston, USA) was used for the statistical analysis. P-values below 0.05 were considered statistically significant.
Results
PAT recordings from 130 patients were analyzed. The participants had a mean age of 53 ± 12 years and 72% (n=93) were male. The average body mass index was 27.6 ± 3.9 kg/m2 and the Epworth Sleepiness Scale was 7.9 ± 5.0. All recordings were automatically and manually scored and a comparison of both scoring methods is given in Table 1. Recording time, heart rate, and oxygen saturation do not require scoring and are therefore constant for both scoring methods.
The mean pAHI of the whole night was 27.5 ± 17.4/h for automated scoring whereas it was only 19.1 ± 15.2/h (p<0.001) for manual scoring. The mean difference of pAHI between automated and manual scoring was 8.4/h. A direct comparison shows that the manually scored recordings lie almost exclusively below the automatically scored data for the whole range of OSA severity (see Fig. 1). The differences between automated and manual scoring are graphically illustrated with a Bland-Altman plot in Fig. 2 and a boxplot in Fig. 3. The lower results for manual scoring were consistent and significant in all sleep stages and body positions (see Table 1). With increasing sleep apnea severity, the absolute difference between automated and manual scoring increased (p<0.001), while the relative difference decreased (p<0.001). For patients with no OSA, the difference was −1.6h (−51%), for mild OSA −4.4/h (−44%), for moderate OSA −8.0/h (−38%), and for severe OSA −11.8/h (−26%). Similarly, a lower pRDI was observed for the manually edited data. The pRDI for the total sleep time was 31.2 ± 16.5/h and 21.7 ± 14.4/h (p<0.001), for automated and manual scoring, respectively.
A Bland-Altman plot comparing automated and manual scoring of peripheral arterial tonometry–derived apnea-hypopnea index (pAHI). The bold line indicates the mean difference between automated and manual scoring, showing lower values for manual scoring (−8.4 events/h). The dashed lines indicate the lower and upper limit of agreement calculated as ±1.96 * the standard deviation
Since ODI is calculated automatically and does not require scoring, differences between the scoring methods are a result of adjustments to the sleep and wake times and the individual sleep stages. Therefore, as expected only minimal, statistically not significant differences in ODI were observed with 15.9 ± 14.5/h for automated and 15.8 ± 13.5/h for manual scoring (p=0.96).
The analysis of pAHI, pRDI, and ODI depending on sleep stage and body position is given in Table 1. Manually scored pAHI and pRDI were significantly lower in all sleep stages and body positions compared to automatically scored data. Surprisingly, the positional OSA, given by the Cartwright index as the ratio between pAHI in supine and non-supine position, increased. The same was observed for REM-associated OSA, given by the ratio between pAHI in REM and NREM sleep, which also increased with manual scoring. These findings indicate that pAHI in non-supine position and NREM sleep decreased more than pAHI in supine and REM sleep.
In our cohort, men had a significantly higher pAHI than women with automated scoring of 30.3±17.2/h and 19.9±15.5/h (p<0.001) and manual scoring of 21.0±15.3/h and 13.9±13.9/h (p=0.003). Manual scoring reduced pAHI in men by −9.7/h (−34.7%) and women by −6.0/h (−36.2%). When accounting for the significantly higher pAHI in men, gender did not significantly influence scoring results (p=0.76). The body mass index category also showed no effect on scoring results (p=0.29, see Supplemental Fig. 1).
Both scoring methods resulted in a similar proportion for all sleep stages with no statistically significant difference. The REM sleep proportion was 24.0±7.1% and 23.6±7.1% (p=0.63) for automated and manual scoring, respectively.
The Pearson’s correlation coefficient of pAHI and ODI increased from 0.88 for automated scoring to 0.94 for manual scoring (p<0.001, see Fig. 4). Similarly, the correlation of pRDI with ODI improved from 0.83 and 0.90, for automated and manual editing, respectively (p<0.001).
Manual editing lead frequently to changes in the OSA category. In only 64 recordings (49%), the category remained the same, whereas it decreased in 64 cases (49%) by one category and in 2 cases (2%) by two categories. No case of an increased OSA category was found.
Discussion
PAT devices are increasingly used as an HSAT to diagnose OSA. Understanding the effect of the scoring method on results is important.
The automated algorithm is well validated and reproducible as well as time- and cost-efficient [11, 12]. The computerized algorithm is also objective, which makes it ideal for clinical studies by eliminating interrater variability. However, it has been demonstrated that this algorithm tends to overestimate respiratory events in patients with less severe OSA when compared to polysomnography [18]. A large cohort study of 500 patients undergoing simultaneous PAT and polysomnography found that PAT overestimated AHI by 4/h compared to polysomnography [19]. Yuceege et al. found AHI for PAT to be significantly higher than polysomnography with a mean difference of 1.78/h [20]. Both studies show an overestimation of respiratory events using PAT, but a lower difference than we observed between automated and manual scoring.
Manual scoring introduces some degree of subjectivity and variability in the analysis of sleep studies. Zhang et al. have developed a manual algorithm to improve the accuracy of both sleep stages and respiratory events [10]. In accordance with their results, we see manual scoring as clinically feasible requiring about 10–15 min to score one recording. The algorithm has been developed in an unselected patient collective, whereas our cohort consisted of patients with suspected sleep-disordered breathing [10].
Our study shows that sleep time and sleep stages are accurately recognized with the automated scoring algorithm of PAT. In the authors’ experience, the automated algorithm for PAT is very accurate in detecting sleep stages with little or no effect added by manual scoring.
However, there are significantly fewer respiratory events in the manually scored recordings. On average, the difference of pAHI was 8.4/h, which frequently resulted in a less severe OSA category. This difference is consistent among all OSA severities, both genders, and all body mass index categories. Zhang et al. found that manual scoring improved accuracy more in women than men [10]. Manual scoring is especially important in patients with mild or moderate OSA, for whom this difference can have implications for treatment recommendation and reimbursement from healthcare insurances.
Manual editing significantly improved the correlation of pAHI and pRDI with ODI in our study, indicating an improved accuracy since the correlation between AHI and ODI has been demonstrated in the literature [15,16,17].
Several limitations need to be mentioned. We did not perform simultaneous recordings with another measuring method, such as polysomnography. Without a direct comparison, it is impossible to describe the accuracy of either scoring method. We used the correlation of ODI with pAHI and pRDI as a surrogate marker for accuracy. This linear relationship has been demonstrated in many publications [15,16,17]. A large study by Ling et al. of more than 11,000 patients demonstrated an increasing ODI/AHI with body mass index [21]. However, since our patients had a narrow distribution of body mass index, we believe that the assumption of a linear relationship is appropriate. Furthermore, Zhang et al. have already demonstrated improved accuracy of manually edited PAT recordings compared to polysomnography in the development of the manual algorithm [10].
A further limitation of our study is that our patient collective was predominantly male and middle aged with a narrow range of body mass index. The recordings were performed in an unselected collective of patients with suspected OSA and retrospectively analyzed. We cannot further characterize our patients by comorbidities or detailed anthropomorphic measurements, because during the pretreatment process only incomplete data were collected. Moreover, the WatchPAT® 200 which was used for all recordings cannot differentiate between central and obstructive respiratory events.
A strength of this study is that all measurements were performed at home in the natural sleeping environment to reflect best the normal sleeping habits of the patients. Patients were instructed to follow their normal nighttime routine and abstain from influencing factors such as sleep medication or alcohol. However, these parameters were not recorded or controlled. To our knowledge, this is the first study to analyze the effect of manual PAT scoring for patients with suspected OSA and in recordings performed at home.
Conclusions
The automated, computer-based algorithm offers a reliable, time- and cost-effective analysis of PAT recordings and eliminates interrater variability. Manual scoring allows for visual oversight over the recording assuring its quality. It also results in significantly lower respiratory event indices but does not significantly affect sleep time and sleep stages. We, therefore, conclude that manual scoring is important for respiratory events and to a lesser degree for sleep stages. Manual scoring might have a larger impact on patients with less severe OSA since treatment recommendations are more likely to change based on the manually scored data. Moreover, manual scoring can affect reimbursement (e.g., mandibular advancement devices), which may be dependent on cut-off values for AHI as it is common in most European countries.
Until improvements to the automated algorithm are implemented and validated, the authors recommend that sleep physicians decide individually if there is a need for manual scoring depending on the clinical situation and the possible impact on decision making.
Abbreviations
- AHI:
-
apnea-hypopnea index
- ENT:
-
ear, nose, and throat
- HSAT:
-
home sleep apnea testing
- NREM:
-
non-rapid eye movement
- ODI:
-
oxygen desaturation index
- OSA:
-
obstructive sleep apnea
- pAHI:
-
peripheral arterial tonometry–derived apnea-hypopnea index
- pRDI:
-
peripheral arterial tonometry–derived respiratory disturbance index
- PAT:
-
peripheral arterial tonometry
- RDI:
-
respiratory disturbance index
- REM:
-
rapid eye movement
References
Remmers JE, deGroot WJ, Sauerland EK, Anch AM (1978) Pathogenesis of upper airway occlusion during sleep. J Appl Physiol 44:931–938. https://doi.org/10.1152/jappl.1978.44.6.931
Gottlieb DJ, Yenokyan G, Newman AB, O’Connor GT, Punjabi NM, Quan SF, Redline S, Resnick HE, Tong EK, Diener-West M, Shahar E (2010) Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation 122:352–360. https://doi.org/10.1161/CIRCULATIONAHA.109.901801
Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V (2005) Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 353:2034–2041. https://doi.org/10.1056/NEJMoa043104
Punjabi NM, Beamer BA (2009) Alterations in glucose disposal in sleep-disordered breathing. Am J Respir Crit Care Med 179:235–240. https://doi.org/10.1164/rccm.200809-1392OC
Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N, Mooser V, Preisig M, Malhotra A, Waeber G, Vollenweider P, Tafti M, Haba-Rubio J (2015) Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med 3:310–318. https://doi.org/10.1016/S2213-2600(15)00043-0
Young T, Evans L, Finn L, Palta M (1997) Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 20:705–706. https://doi.org/10.1093/sleep/20.9.705
American Academy of Sleep Medicine (2020) The American Academy of Sleep Medicine manual for the scoring of sleep and associated events version 2.6. https://aasm.org/clinical-resources/scoring-manual/
Tschopp S, Wimmer W, Caversaccio M, Borner U, Tschopp K (2021) Night-to-night variability in obstructive sleep apnea using peripheral arterial tonometry: a case for multiple night testing. J Clin Sleep Med jcsm.9300:jcsm.9300. https://doi.org/10.5664/jcsm.9300
Garg N, Rolle AJ, Lee TA, Prasad B (2014) Home-based diagnosis of obstructive sleep apnea in an urban population. J Clin Sleep Med 10:879–885. https://doi.org/10.5664/jcsm.3960
Zhang Z, Sowho M, Otvos T, Sperandio LS, East J, Sgambati F, Schwartz A, Schneider H (2020) A comparison of automated and manual sleep staging and respiratory event recognition in a portable sleep diagnostic device with in-lab sleep study. J Clin Sleep Med 16:563–573. https://doi.org/10.5664/jcsm.8278
Yalamanchali S, Farajian V, Hamilton C, Pott TR, Samuelson CG, Friedman M (2013) Diagnosis of obstructive sleep apnea by peripheral arterial tonometry: meta-analysis. JAMA Otolaryngol Head Neck Surg 139:1343. https://doi.org/10.1001/jamaoto.2013.5338
Hedner J, White DP, Malhotra A, Herscovici S, Pittman SD, Zou D, Grote L, Pillar G (2011) Sleep staging based on autonomic signals: a multi-center validation study. J Clin Sleep Med 07:301–306. https://doi.org/10.5664/JCSM.1078
Cartwright RD (1984) Effect of sleep position on sleep apnea severity. Sleep 7:110–114. https://doi.org/10.1093/sleep/7.2.110
Oksenberg A, Arons E, Nasser K, Vander T, Radwan H (2010) REM-related obstructive sleep apnea: the effect of body position. J Clin Sleep Med 6:343–348
Chung F, Liao P, Elsaid H, Islam S, Shapiro CM, Sun Y (2012) Oxygen desaturation index from nocturnal oximetry: a sensitive and specific tool to detect sleep-disordered breathing in surgical patients. Anesth Analg 114:993–1000. https://doi.org/10.1213/ANE.0b013e318248f4f5
Ernst G, Bosio M, Salvado A, Dibur E, Nigro C, Borsini E (2016) Difference between apnea-hypopnea index (AHI) and oxygen desaturation index (ODI): proportional increase associated with degree of obesity. Sleep Breath 20:1175–1183. https://doi.org/10.1007/s11325-016-1330-3
Dawson A, Loving RT, Gordon RM, Abel SL, Loewy D, Kripke DF, Kline LE (2015) Type III home sleep testing versus pulse oximetry: is the respiratory disturbance index better than the oxygen desaturation index to predict the apnoea-hypopnoea index measured during laboratory polysomnography? BMJ Open 5:e007956. https://doi.org/10.1136/bmjopen-2015-007956
Ayas N (2003) Assessment of a wrist-worn device in the detection of obstructive sleep apnea. Sleep Med 4:435–442. https://doi.org/10.1016/S1389-9457(03)00111-4
Ioachimescu OC, Allam JS, Samarghandi A, Anand N, Fields BG, Dholakia SA, Venkateshiah SB, Eisenstein R, Ciavatta M-M, Collop NA, for the Pulse Arterial Tonometry Evaluation of Reliability (PATER) study investigators (2020) Performance of peripheral arterial tonometry–based testing for the diagnosis of obstructive sleep apnea in a large sleep clinic cohort. J Clin Sleep Med 16:1663–1674. https://doi.org/10.5664/jcsm.8620
Yuceege M, Firat H, Demir A, Ardic S (2013) Reliability of the Watch-PAT 200 in detecting sleep apnea in highway bus drivers. J Clin Sleep Med 09:339–344. https://doi.org/10.5664/jcsm.2584
Ling IT, James AL, Hillman DR (2012) Interrelationships between body mass, oxygen desaturation, and apnea-hypopnea indices in a sleep clinic population. Sleep 35:89–96. https://doi.org/10.5665/sleep.1592
Funding
Open access funding provided by University of Bern
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation and data collection were performed by Samuel Tschopp and Kurt Tschopp. The analysis was performed by Wilhelm Wimmer, Samuel Tschopp, Urs Borner, Kurt Tschopp, and Marco Caversaccio. The first draft was written by Samuel Tschopp. All authors commented on the previous versions of the manuscript. All authors have read and approved the final version of this manuscript.
Corresponding author
Ethics declarations
Ethics approval
EKNZ 2018-01579.
Consent to participate
Given by all participants by informed consent.
Consent to publish
Given by all participants by informed consent.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information

ESM 1
Supplemental Fig. 1 Difference between automated and manual scoring in peripheral arterial tonometry-derived apnea-hypopnea index (pAHI) by gender (a) and by body mass index (b). When accounting for sleep apnea severity, no statistically significant difference lies between the gender (p=0.76) or body mass index categories (p=0.29) (PNG 60 kb)

ESM 2
(PNG 67 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tschopp, S., Borner, U., Wimmer, W. et al. Clinical impact of manual scoring of peripheral arterial tonometry in patients with sleep apnea. Sleep Breath 27, 229–237 (2023). https://doi.org/10.1007/s11325-021-02531-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-021-02531-9